Botulinum neurotoxin type A in the interdisciplinary treatment of sialorrhea in adults and children-update and practice recommendations
- PMID: 38162447
- PMCID: PMC10757066
- DOI: 10.3389/fneur.2023.1275807
Botulinum neurotoxin type A in the interdisciplinary treatment of sialorrhea in adults and children-update and practice recommendations
Abstract
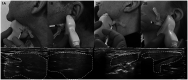
Sialorrhea is defined as a chronic excessive flow of saliva from the mouth, often with adverse consequences for health and quality of life of patients. In addition to currently used non-drug treatment and systemic drugs, intraglandular Botulinum Neurotoxin A (BoNT/A) injections have been examined in case studies, controlled trials and clinical practice. Two pivotal Phase III trials recently led to market approval in the USA and EU for IncobotulinumtoxinA [Xeomin®, IncoBoNT/A, Clostridium botulinum neurotoxin type A (150 kD), free from complexing proteins, Merz Pharmaceuticals GmbH] for treatment of chronic sialorrhea in adults and pediatric patients. This review provides a multidisciplinary approach to discuss the current state of sialorrhea therapy as well as benefits and current limitations of BoNT/A injections. A consensus regarding treatment recommendations made available to physicians in Germany in 2022 has now been updated here for presentation to an international audience. This review provides a framework including a flow chart for patient selection, recommendations for dosing and the injection process, as well as a discussion of therapeutic goals, long-term benefits and safety aspects. This review is aimed at supporting physicians in developing multidisciplinary and individualized treatment approaches to achieve optimal benefits for patients.
Keywords: BoNT/A; IncobotulinumtoxinA; botulinum neurotoxin type A; botulinum toxin; hypersalivation; practice recommendations; quality of life; sialorrhea.
Copyright © 2023 Jost, Bäumer, Bevot, Birkmann, Buhmann, Grosheva, Guntinas-Lichius, Laskawi, Paus, Pflug, Schroeder, Spittau, Steffen, Wilken, Winterholler and Berweck.
Conflict of interest statement
The content of this review was created based on interdisciplinary consultations and cooperation with all authors and the consensus recommendations were developed during three working meetings. Merz Therapeutics GmbH supported these meetings financially but had no further influence on the recommendations and the content of this review. WJ is or was a consultant and speaker for AbbVie, Bial, Desitin, Kyowa Kirin, Stada, UCB, Zambon, Pharm Allergan, Ipsen Pharma, and Merz Therapeutics and was principal investigator of the SIAXI study. TB is a consultant for Merz Therapeutics and Pharm Allergan and a speaker for Merz Therapeutics, Pharm Allergan, and Ipsen. He received speaker and consultant fees from the Pelzerhaken Children’s Center, AbbVie/Allergan, Ipsen Pharma, and Merz Therapeutics. He received research grants from Deutsche Forschungsgemeinschaft (FG2698-2) and Ipsen Pharma. AB received consultant and speaker fees from Allergan/AbbVie, Merz Therapeutics, and Novartis. UB received consultant fees from Merz Therapeutics. CB received speaker and/or consultant fees from AbbVie, Bial, Desitin, Kyowa Kirin, Merz Therapeutics, STADA Pharm, TAD Pharma, UCB, and Zambon. MG received speaker and consultant fees from Merz Therapeutics, MSD, and Bayer Vital. OG-L received speaker and consultant fees from Merz Therapeutics, MEDICE, MEDEL, and Novartis. RL is a speaker for Merz Therapeutics. SP is or was a consultant for Bial, Idorsia, Ipsen, Merz Therapeutics, and TEVA and a speaker from AbbVie, Bayer, Bial, Ipsen, Merz Therapeutics, and Novartis. Furthermore, SP is or was involved in clinical studies (phase II and III) for Bayer, Ipsen, and Syneos Health. CP received honoraria as a speaker and/or consultant from AbbVie, Merz Therapeutics, InfectoPharm, and Olympus. ASc was a consultant and speaker for Allergan/AbbVie, Ipsen, Merz Therapeutics, Proveca, and Medice. ASt is a consultant and speaker for Merz Therapeutics, Inspire Medical, and ZOLL Respicardia. He received speaking honoraria and travel support from Intersect Medical and Clinigen. BW received speaker fees from AbbVie/Allergan, Takeda, Novartis, and Eisai. MW received honoraria as a speaker and/or consultant from AbbVie. SB received speaker fees from Merz Therapeutics, Ipsen Pharma, and Pharm Allergan and consultant fees from Merz Therapeutics and Ipsen Pharma. He was principal investigator of the SIPEXI study. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

