Urine-based point-of-care testing for factor-Xa-inhibitors in acute ischemic stroke patients: a feasibility study
- PMID: 38162451
- PMCID: PMC10756232
- DOI: 10.3389/fneur.2023.1330421
Urine-based point-of-care testing for factor-Xa-inhibitors in acute ischemic stroke patients: a feasibility study
Abstract
Introduction: Direct oral anticoagulants (DOACs) have become widely used in clinical practice for preventing thromboembolic events. Point-of-care testing methods, particularly those based on urine samples, offer a promising approach for rapid and accurate assessment of DOAC presence. This pilot study aims to evaluate the utility of a urine-based DOAC dipstick test as a point-of-care tool for identifying DOAB presence in acute ischemic stroke (AIS) or transient ischemic attack (TIA) patients.
Patients and methods: This prospective pilot study included patients with AIS/TIA eligible for DOAC-measurement. After exclusion of 3 patients, 23 patients with DOAC-intake (DOAC group; factor-Xa-inhibitors; n = 23) and 21 patients without DOAC-intake (control-group) remained for analyses. The urine-based DOAC dipstick test and parallel blood-based specific DOAC-level assessment were performed in all patients. Time-intervals of sampling urine/blood sampling and result of DOAC-test were recorded to analyze a potential time benefit based on dipstick evaluation.
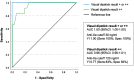
Results: The urine-based DOAC dipstick test demonstrated high sensitivity (100%) and specificity (100%), correctly identifying all patients with anticoagulatory activity due to DOAC intake (i.e., anti-Xalevel ≥30 ng/mL). Moreover, the visual readout of the test provided semiquantitative information on drug-specific anti-Xa levels, showing a sensitivity of 83% and specificity of 93% to detect anti-Xa levels ≥120 ng/mL. The dipstick test exhibited a median time-benefit of 2:25 h compared to standard blood-based DOAC-level testing.
Discussion: The results of this pilot study underline the efficacy of urine-based point-of-care testing as a rapid and reliable method for assessing DOAC presence in patients with acute ischemic stroke.
Conclusion: The value of this tool for clinical decision-making in stroke management needs to be established in future trials.Clinical Trial Registration: Clinicaltrails.org identifier [NCT06037200].
Keywords: anticoagulation; direct oral anticoagulant; factor-Xa-inhibitor; ischemic stroke; point-of-care test.
Copyright © 2023 Doeppner, Olbricht, Maxhuni, Alhaj Omar, Sachs, Juenemann, Huttner and Gerner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/s0140-6736(13)62343-0, PMID: - DOI - PubMed
-
- Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, et al. . International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. (2017) 194:132–40. doi: 10.1016/j.ahj.2017.08.011, PMID: - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

