Risk of severe COVID-19 outcomes after autumn 2022 COVID-19 booster vaccinations: a pooled analysis of national prospective cohort studies involving 7.4 million adults in England, Northern Ireland, Scotland and Wales
- PMID: 38162515
- PMCID: PMC10757260
- DOI: 10.1016/j.lanepe.2023.100816
Risk of severe COVID-19 outcomes after autumn 2022 COVID-19 booster vaccinations: a pooled analysis of national prospective cohort studies involving 7.4 million adults in England, Northern Ireland, Scotland and Wales
Abstract
Background: UK COVID-19 vaccination policy has evolved to offering COVID-19 booster doses to individuals at increased risk of severe Illness from COVID-19. Building on our analyses of vaccine effectiveness of first, second and initial booster doses, we aimed to identify individuals at increased risk of severe outcomes (i.e., COVID-19 related hospitalisation or death) post the autumn 2022 booster dose.
Methods: We undertook a national population-based cohort analysis across all four UK nations through linked primary care, vaccination, hospitalisation and mortality data. We included individuals who received autumn 2022 booster doses of BNT162b2 (Comirnaty) or mRNA-1273 (Spikevax) during the period September 1, 2022 to December 31, 2022 to investigate the risk of severe COVID-19 outcomes. Cox proportional hazard models were used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CIs) for the association between demographic and clinical factors and severe COVID-19 outcomes after the autumn booster dose. Analyses were adjusted for age, sex, body mass index (BMI), deprivation, urban/rural areas and comorbidities. Stratified analyses were conducted by vaccine type. We then conducted a fixed-effect meta-analysis to combine results across the four UK nations.
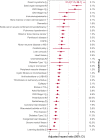
Findings: Between September 1, 2022 and December 31, 2022, 7,451,890 individuals ≥18 years received an autumn booster dose. 3500 had severe COVID-19 outcomes (2.9 events per 1000 person-years). Being male (male vs female, aHR 1.41 (1.32-1.51)), older adults (≥80 years vs 18-49 years; 10.43 (8.06-13.50)), underweight (BMI <18.5 vs BMI 25.0-29.9; 2.94 (2.51-3.44)), those with comorbidities (≥5 comorbidities vs none; 9.45 (8.15-10.96)) had a higher risk of COVID-19 hospitalisation or death after the autumn booster dose. Those with a larger household size (≥11 people within household vs 2 people; 1.56 (1.23-1.98)) and from more deprived areas (most deprived vs least deprived quintile; 1.35 (1.21-1.51)) had modestly higher risks. We also observed at least a two-fold increase in risk for those with various chronic neurological conditions, including Down's syndrome, immunodeficiency, chronic kidney disease, cancer, chronic respiratory disease, or cardiovascular disease.
Interpretation: Males, older individuals, underweight individuals, those with an increasing number of comorbidities, from a larger household or more deprived areas, and those with specific underlying health conditions remained at increased risk of COVID-19 hospitalisation and death after the autumn 2022 vaccine booster dose. There is now a need to focus on these risk groups for investigating immunogenicity and efficacy of further booster doses or therapeutics.
Funding: National Core Studies-Immunity, UK Research and Innovation (Medical Research Council and Economic and Social Research Council), Health Data Research UK, the Scottish Government, and the University of Edinburgh.
Keywords: Booster dose; COVID-19; Hospital admission; Vaccine; Vaccine breakthrough.
© 2023 The Authors.
Conflict of interest statement
AS and CR are members of the Scottish Government's CMO COVID-19 Advisory Group. AS and CR are members of NERVTAG's risk stratification subgroup. CR is a member of SPI-M. AS is a member of AstraZeneca's Thrombotic Thrombocytopenic Advisory Group and the Scottish Government's Standing Committee on Pandemics. All roles are unremunerated. RAL is a member of the Welsh Government COVID-19 Technical Advisory Group. All other co-authors report no conflict of interests. SdeL is Director of the RSC, through his university he has received vaccine related research funding from AstraZeneca, GSK, Sanofi, Seqirus, MSD and Takeda, and been member of advisory boards for AstraZeneca, GSK, Sanofi and Seqirus.
Figures

Similar articles
-
Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales.Lancet. 2022 Oct 15;400(10360):1305-1320. doi: 10.1016/S0140-6736(22)01656-7. Lancet. 2022. PMID: 36244382 Free PMC article.
-
COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study.Lancet Respir Med. 2021 Dec;9(12):1439-1449. doi: 10.1016/S2213-2600(21)00380-5. Epub 2021 Sep 29. Lancet Respir Med. 2021. PMID: 34599903 Free PMC article.
-
Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study.Lancet Infect Dis. 2022 Jan;22(1):43-55. doi: 10.1016/S1473-3099(21)00460-6. Epub 2021 Sep 1. Lancet Infect Dis. 2022. PMID: 34480857 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The effectiveness of the first dose COVID-19 booster vs. full vaccination to prevent SARS-CoV-2 infection and severe COVID-19 clinical event: a meta-analysis and systematic review of longitudinal studies.Front Public Health. 2023 Jun 1;11:1165611. doi: 10.3389/fpubh.2023.1165611. eCollection 2023. Front Public Health. 2023. PMID: 37325336 Free PMC article.
Cited by
-
History of childhood maltreatment associated with hospitalization or death due to COVID-19: a cohort study.BMC Med. 2024 Aug 7;22(1):319. doi: 10.1186/s12916-024-03399-8. BMC Med. 2024. PMID: 39113083 Free PMC article.
-
Is Metformin Use Associated with a More Favorable COVID-19 Course in People with Diabetes?J Clin Med. 2024 Mar 24;13(7):1874. doi: 10.3390/jcm13071874. J Clin Med. 2024. PMID: 38610639 Free PMC article.
-
The Role of COVID-19 in Excess Mortality in Slovakia: A Novel Approach Based on Healthcare Billing Records.Int J Public Health. 2024 Dec 3;69:1607537. doi: 10.3389/ijph.2024.1607537. eCollection 2024. Int J Public Health. 2024. PMID: 39691547 Free PMC article.
-
Enabling health data analyses across multiple private datasets with no information sharing using secure multiparty computation.BMJ Health Care Inform. 2025 May 26;32(1):e101384. doi: 10.1136/bmjhci-2024-101384. BMJ Health Care Inform. 2025. PMID: 40425203 Free PMC article.
-
Epidemiology, Immunology and Clinical Characteristics of COVID-19 (EPIC3)-Database of a prospective longitudinal observational study within the Veterans Health Administration.Front Public Health. 2025 Jun 2;13:1535315. doi: 10.3389/fpubh.2025.1535315. eCollection 2025. Front Public Health. 2025. PMID: 40538697 Free PMC article. No abstract available.
References
-
- UK Department of Health & Social Care . 2022. JCVI statement on the COVID-19 booster vaccination programme for autumn 2022: update 3 September 2022.https://www.gov.uk/government/publications/covid-19-vaccines-for-autumn-...
-
- Chae C., Kim R.K., Jang E.J., et al. Comparing the effectiveness of bivalent and monovalent COVID-19 vaccines against COVID-19 infection during the winter season of 2022-2023: a real-world retrospective observational matched cohort study in the Republic of Korea. Int J Infect Dis. 2023;135:95–100. - PubMed
-
- Tan C.Y., Chiew C.J., Pang D., et al. Effectiveness of bivalent mRNA vaccines against medically attended symptomatic SARS-CoV-2 infection and COVID-19-related hospital admission among SARS-CoV-2-naive and previously infected individuals: a retrospective cohort study. Lancet Infect Dis. 2023;23:1343. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

