Monitoring time domain characteristics of Parkinson's disease using 3D memristive neuromorphic system
- PMID: 38162516
- PMCID: PMC10754992
- DOI: 10.3389/fncom.2023.1274575
Monitoring time domain characteristics of Parkinson's disease using 3D memristive neuromorphic system
Abstract
Introduction: Parkinson's disease (PD) is a neurodegenerative disorder affecting millions of patients. Closed-Loop Deep Brain Stimulation (CL-DBS) is a therapy that can alleviate the symptoms of PD. The CL-DBS system consists of an electrode sending electrical stimulation signals to a specific region of the brain and a battery-powered stimulator implanted in the chest. The electrical stimuli in CL-DBS systems need to be adjusted in real-time in accordance with the state of PD symptoms. Therefore, fast and precise monitoring of PD symptoms is a critical function for CL-DBS systems. However, the current CL-DBS techniques suffer from high computational demands for real-time PD symptom monitoring, which are not feasible for implanted and wearable medical devices.
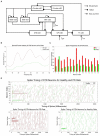
Methods: In this paper, we present an energy-efficient neuromorphic PD symptom detector using memristive three-dimensional integrated circuits (3D-ICs). The excessive oscillation at beta frequencies (13-35 Hz) at the subthalamic nucleus (STN) is used as a biomarker of PD symptoms.
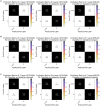
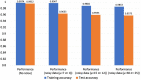
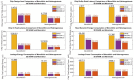
Results: Simulation results demonstrate that our neuromorphic PD detector, implemented with an 8-layer spiking Long Short-Term Memory (S-LSTM), excels in recognizing PD symptoms, achieving a training accuracy of 99.74% and a validation accuracy of 99.52% for a 75%-25% data split. Furthermore, we evaluated the improvement of our neuromorphic CL-DBS detector using NeuroSIM. The chip area, latency, energy, and power consumption of our CL-DBS detector were reduced by 47.4%, 66.63%, 65.6%, and 67.5%, respectively, for monolithic 3D-ICs. Similarly, for heterogeneous 3D-ICs, employing memristive synapses to replace traditional Static Random Access Memory (SRAM) resulted in reductions of 44.8%, 64.75%, 65.28%, and 67.7% in chip area, latency, and power usage.
Discussion: This study introduces a novel approach for PD symptom evaluation by directly utilizing spiking signals from neural activities in the time domain. This method significantly reduces the time and energy required for signal conversion compared to traditional frequency domain approaches. The study pioneers the use of neuromorphic computing and memristors in designing CL-DBS systems, surpassing SRAM-based designs in chip design area, latency, and energy efficiency. Lastly, the proposed neuromorphic PD detector demonstrates high resilience to timing variations in brain neural signals, as confirmed by robustness analysis.
Keywords: Parkinson’s disease; deep brain stimulation; memristors; neuromorphic computing; spiking neural networks.
Copyright © 2023 Siddique, Zhang and An.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Closed-Loop Deep Brain Stimulation Effects on Parkinsonian Motor Symptoms in a Non-Human Primate - Is Beta Enough?Brain Stimul. 2016 Nov-Dec;9(6):892-896. doi: 10.1016/j.brs.2016.06.051. Epub 2016 Jun 22. Brain Stimul. 2016. PMID: 27401045 Free PMC article.
-
Mapping Motor Pathways in Parkinson's Disease Patients with Subthalamic Deep Brain Stimulator: A Diffusion MRI Tractography Study.Neurol Ther. 2022 Jun;11(2):659-677. doi: 10.1007/s40120-022-00331-1. Epub 2022 Feb 14. Neurol Ther. 2022. PMID: 35165822 Free PMC article.
-
Three-dimensional SPACE fluid-attenuated inversion recovery at 3 T to improve subthalamic nucleus lead placement for deep brain stimulation in Parkinson's disease: from preclinical to clinical studies.J Neurosurg. 2016 Aug;125(2):472-80. doi: 10.3171/2015.7.JNS15379. Epub 2016 Jan 8. J Neurosurg. 2016. PMID: 26745490
-
Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations.Mov Disord. 2017 Jan;32(1):80-88. doi: 10.1002/mds.26837. Epub 2016 Nov 8. Mov Disord. 2017. PMID: 27859579 Review.
-
Alternate Subthalamic Nucleus Deep Brain Stimulation Parameters to Manage Motor Symptoms of Parkinson's Disease: Systematic Review and Meta-analysis.Mov Disord Clin Pract. 2018 Nov 8;6(1):17-26. doi: 10.1002/mdc3.12681. eCollection 2019 Jan. Mov Disord Clin Pract. 2018. PMID: 30746411 Free PMC article. Review.
Cited by
-
A robust Parkinson's disease detection model based on time-varying synaptic efficacy function in spiking neural network.BMC Neurol. 2024 Dec 30;24(1):492. doi: 10.1186/s12883-024-04001-7. BMC Neurol. 2024. PMID: 39734199 Free PMC article.
References
-
- Akopyan F., Sawada J., Cassidy A., Alvarez-Icaza R., Arthur J., Merolla P., et al. . (2015). TrueNorth: design and tool flow of a 65 mW 1 million neuron programmable neurosynaptic chip. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 34, 1537–1557. doi: 10.1109/TCAD.2015.2474396 - DOI
-
- An H. (2020). “Powering next-generation artificial intelligence by designing three-dimensional high-performance neuromorphic computing system with memristors” in Dissertation (Blacksburg, VA: Virginia Tech; ).
-
- An H., Al-Mamun M. S., Orlowski M. K., Yi Y. (2018a). Learning accuracy analysis of memristor-based nonlinear computing module on long short-term memory. Proceedings of the International Conference on Neuromorphic Systems.
LinkOut - more resources
Full Text Sources

