Inactivated vaccine effectiveness against symptomatic COVID-19 in Fujian, China during the Omicron BA.2 outbreak
- PMID: 38162626
- PMCID: PMC10757624
- DOI: 10.3389/fpubh.2023.1269194
Inactivated vaccine effectiveness against symptomatic COVID-19 in Fujian, China during the Omicron BA.2 outbreak
Abstract
Objective: More than 90% of the Chinese population have completed 2 doses of inactivated COVID-19 vaccines in Mainland China. However, after China government abandoned strict control measures, many breakthrough infections appeared, and vaccine effectiveness against Omicron BA.2 infection was uncertain. This study aims to investigate the real-world effectiveness of widely used inactivated vaccines during the wave of Omicron variants.
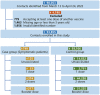
Methods: Test-negative case-control study was conducted in this study to analyze the vaccine effectiveness against symptomatic disease caused by the Omicron variant (BA.2) in Fujian, China. Conditional logistic regression was selected to estimate the vaccine effectiveness.
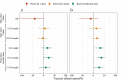
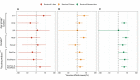
Results: The study found the vaccine effectiveness against symptomatic COVID-19 is 32.46% (95% CI, 8.08% to 50.37%) at 2 to 8 weeks, and 27.05% (95% CI, 1.23% to 46.12%) at 12 to 24 weeks after receiving booster doses of the inactivated vaccine. Notably, the 3-17 years group had higher vaccine effectiveness after 2 doses than the 18-64 years and over 65 years groups who received booster doses.
Conclusion: Inactivated vaccines alone may not offer sufficient protection for all age groups before the summer of 2022. To enhance protection, other types of vaccines or bivalent vaccines should be considered.
Keywords: COVID-19; Omicron BA.2; SARS-CoV-2; inactivated vaccine; vaccine effectiveness (VE).
Copyright © 2023 Ye, Li, Zhao, Wu, Qu, Guo, Abudunaibi, Chen, Cai, Chen, Lin, Xie, Zhan, Ou, Deng, Chen and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effectiveness of a booster dose of COVID-19 vaccines during an outbreak of SARS-CoV-2 Omicron BA.2.2 in China: A case-control study.Hum Vaccin Immunother. 2023 Dec 31;19(1):2194189. doi: 10.1080/21645515.2023.2194189. Epub 2023 Mar 30. Hum Vaccin Immunother. 2023. PMID: 36998173 Free PMC article.
-
Effectiveness of the booster dose of inactivated COVID-19 vaccine against Omicron BA.5 infection: a matched cohort study of adult close contacts.Respir Res. 2023 Oct 12;24(1):246. doi: 10.1186/s12931-023-02542-y. Respir Res. 2023. PMID: 37828565 Free PMC article.
-
Transmission Characteristics and Inactivated Vaccine Effectiveness Against Transmission of SARS-CoV-2 Omicron BA.5 Variants in Urumqi, China.JAMA Netw Open. 2023 Mar 1;6(3):e235755. doi: 10.1001/jamanetworkopen.2023.5755. JAMA Netw Open. 2023. PMID: 36995713 Free PMC article.
-
Real-world effectiveness and factors associated with effectiveness of inactivated SARS-CoV-2 vaccines: a systematic review and meta-regression analysis.BMC Med. 2023 Apr 27;21(1):160. doi: 10.1186/s12916-023-02861-3. BMC Med. 2023. PMID: 37106390 Free PMC article.
-
Real-world evidence on the efficacy of bivalent booster doses of SARS-CoV-2 vaccine in respect of monovalent boosters or primary cycle of vaccination: a narrative review.Epidemiol Prev. 2023 Nov-Dec;47(6):331-343. doi: 10.19191/EP23.6.A626.081. Epidemiol Prev. 2023. PMID: 38314543 Review. English.
Cited by
-
Impacts of COVID-19 vaccine boosters on clinical outcomes associated with the Omicron variant in China: A cross-sectional survey.Vaccine X. 2024 Jun 6;19:100508. doi: 10.1016/j.jvacx.2024.100508. eCollection 2024 Aug. Vaccine X. 2024. PMID: 38903607 Free PMC article.
-
Immunogen characterization reveals an intrinsic hindrance in eliciting neutralizing antibodies against JN.1 variant.iScience. 2024 Jun 28;27(8):110405. doi: 10.1016/j.isci.2024.110405. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108735 Free PMC article.
References
-
- Fu Y, Zhao J, Wei X, Han P, Yang L, Ren T, et al. . Effectiveness and cost-effectiveness of inactivated vaccine to address COVID-19 pandemic in China: evidence from randomized control trials and real-world studies. Front Public Health. (2022) 10:917732. 10.3389/fpubh.2022.917732 - DOI - PMC - PubMed
-
- Wan EY, Mok AH, Yan VK, Wang B, Zhang R, Hong SN, et al. . Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: A case control study. J Infect. (2022) 85:e140–4. 10.1016/j.jinf.2022.08.008 - DOI - PMC - PubMed
-
- Prc N. H. C. Diagnosis and treatment plan for COVID-19 (Trial Version 9). (2022). Available online at: http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm (accessed November 10, 2023).
-
- World Health Organization . Enhancing response to Omicron SARS-CoV-2 variant: Technical brief and priority actions for Member States. Geneva, Switzerland: World Health Organization; (2022). Available online at: https://www.who.int/docs/default-source/coronaviruse/2022-01-21-global-t... (accessed November 10, 2023).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

