The role of immune checkpoints in antitumor response: a potential antitumor immunotherapy
- PMID: 38162657
- PMCID: PMC10757365
- DOI: 10.3389/fimmu.2023.1298571
The role of immune checkpoints in antitumor response: a potential antitumor immunotherapy
Abstract
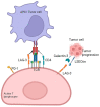
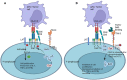
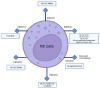
Immunotherapy aims to stimulate the immune system to inhibit tumor growth or prevent metastases. Tumor cells primarily employ altered expression of human leukocyte antigen (HLA) as a mechanism to avoid immune recognition and antitumor immune response. The antitumor immune response is primarily mediated by CD8+ cytotoxic T cells (CTLs) and natural killer (NK) cells, which plays a key role in the overall anti-tumor immune response. It is crucial to comprehend the molecular events occurring during the activation and subsequent regulation of these cell populations. The interaction between antigenic peptides presented on HLA-I molecules and the T-cell receptor (TCR) constitutes the initial signal required for T cell activation. Once activated, in physiologic circumstances, immune checkpoint expression by T cells suppress T cell effector functions when the antigen is removed, to ensures the maintenance of self-tolerance, immune homeostasis, and prevention of autoimmunity. However, in cancer, the overexpression of these molecules represents a common method through which tumor cells evade immune surveillance. Numerous therapeutic antibodies have been developed to inhibit immune checkpoints, demonstrating antitumor activity with fewer side effects compared to traditional chemotherapy. Nevertheless, it's worth noting that many immune checkpoint expressions occur after T cell activation and consequently, altered HLA expression on tumor cells could diminish the clinical efficacy of these antibodies. This review provides an in-depth exploration of immune checkpoint molecules, their corresponding blocking antibodies, and their clinical applications.
Keywords: HLA antigens; immune checkpoint inhibitors; immune evasion; immunotherapy; neoplasms.
Copyright © 2023 Mejía-Guarnizo, Monroy-Camacho, Turizo-Smith and Rodríguez-García.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Re-targeting of cytotoxic T lymphocytes and/or natural killer cells to CEA-expressing tumor cells with anti-CEA antibody activity.Anticancer Res. 2005 Nov-Dec;25(6A):3725-32. Anticancer Res. 2005. PMID: 16302732 Review.
-
Enhancing Natural Killer and CD8+ T Cell-Mediated Anticancer Cytotoxicity and Proliferation of CD8+ T Cells with HLA-E Monospecific Monoclonal Antibodies.Monoclon Antib Immunodiagn Immunother. 2019 Apr;38(2):38-59. doi: 10.1089/mab.2018.0043. Monoclon Antib Immunodiagn Immunother. 2019. PMID: 31009335 Free PMC article. Review.
-
The analysis of the natural killer-like activity of human cytolytic T lymphocytes revealed HLA-E as a novel target for TCR alpha/beta-mediated recognition.Eur J Immunol. 2001 Dec;31(12):3687-93. doi: 10.1002/1521-4141(200112)31:12<3687::aid-immu3687>3.0.co;2-c. Eur J Immunol. 2001. PMID: 11745389
-
Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas.Gastroenterology. 2017 Oct;153(4):1107-1119.e10. doi: 10.1053/j.gastro.2017.06.017. Epub 2017 Jun 23. Gastroenterology. 2017. PMID: 28648905
-
Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells.Front Immunol. 2018 Sep 10;9:2041. doi: 10.3389/fimmu.2018.02041. eCollection 2018. Front Immunol. 2018. PMID: 30250471 Free PMC article. Review.
Cited by
-
Tumor Microenvironment Drives the Cross-Talk Between Co-Stimulatory and Inhibitory Molecules in Tumor-Infiltrating Lymphocytes: Implications for Optimizing Immunotherapy Outcomes.Int J Mol Sci. 2024 Nov 29;25(23):12848. doi: 10.3390/ijms252312848. Int J Mol Sci. 2024. PMID: 39684559 Free PMC article. Review.
-
Cracking Chordoma's Conundrum: Immune Checkpoints Provide a Potential Modality.Int J Med Sci. 2025 Apr 22;22(10):2318-2332. doi: 10.7150/ijms.109721. eCollection 2025. Int J Med Sci. 2025. PMID: 40386066 Free PMC article.
-
Combination Therapy Approaches to Enhance the Efficacy of ERV-Targeting Vaccines in Cancer.Cancer Immunol Res. 2025 Jun 4;13(6):792-803. doi: 10.1158/2326-6066.CIR-24-1192. Cancer Immunol Res. 2025. PMID: 40387511 Free PMC article. Review.
-
γδT cells, a key subset of T cell for cancer immunotherapy.Front Immunol. 2025 Mar 28;16:1562188. doi: 10.3389/fimmu.2025.1562188. eCollection 2025. Front Immunol. 2025. PMID: 40226616 Free PMC article. Review.
-
Immune evasion in cancer: mechanisms and cutting-edge therapeutic approaches.Signal Transduct Target Ther. 2025 Jul 31;10(1):227. doi: 10.1038/s41392-025-02280-1. Signal Transduct Target Ther. 2025. PMID: 40739089 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

