IL-17A-driven psoriasis is critically dependent on IL-36 signaling
- PMID: 38162658
- PMCID: PMC10754973
- DOI: 10.3389/fimmu.2023.1256133
IL-17A-driven psoriasis is critically dependent on IL-36 signaling
Abstract
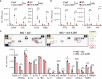
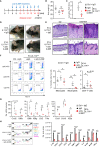
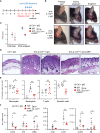
Plaque psoriasis is an autoinflammatory and autoimmune skin disease, affecting 1-3% of the population worldwide. Previously, high levels of IL-36 family cytokines were found in psoriatic skin lesions, thereby contributing to keratinocyte hyperproliferation and infiltration of immune cells such as neutrophils. While treatment with anti-IL36 receptor (IL36R) antibodies was recently approved for generalized pustular psoriasis (GPP), it remains unclear, if targeting the IL36R might also inhibit plaque psoriasis. Here we show that antibody-mediated inhibition of IL36R is sufficient to suppress imiquimod-induced psoriasis-like skin inflammation and represses the disease's development in a model that depends on IL-17A overexpression in the skin. Importantly, treatment with anti-IL36R antibodies inhibited skin inflammation and attenuated psoriasis-associated, systemic inflammation. This is possibly due to a widespread effect of IL36R inhibition, which not only suppresses pro-inflammatory gene expression in keratinocytes, but also the activation of other immune cells such as T-cells or dendritic cells. In conclusion, we propose that inhibition of the IL-36 signaling pathway might constitute an attractive, alternative approach for treating IL-17A-driven psoriasis and psoriasis-linked comorbidities.
Keywords: IL-17A; anti-IL36R; imiquimod; keratinocytes; psoriasis; spesolimab; systemic inflammation.
Copyright © 2023 Fischer, Kübelbeck, Kolb, Ringen, Waisman, Wittmann, Karbach, Kölsch and Kramer.
Conflict of interest statement
SMK is employed by Boehringer Ingelheim Pharma GmbH & Co. KG. SK declares having received consultancy honorary from Almirall and lecture honoraria from Janssen-Cilag, which both was not associated with this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Boutet MA, Bart G, Penhoat M, Amiaud J, Brulin B, Charrier C, et al. . Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and crohn's disease. Clin Exp Immunol (2016) 184(2):159–73. doi: 10.1111/cei.12761 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

