Bioavailable central nervous system disease-modifying therapies for multiple sclerosis
- PMID: 38162670
- PMCID: PMC10755740
- DOI: 10.3389/fimmu.2023.1290666
Bioavailable central nervous system disease-modifying therapies for multiple sclerosis
Abstract
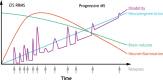
Disease-modifying therapies for relapsing multiple sclerosis reduce relapse rates by suppressing peripheral immune cells but have limited efficacy in progressive forms of the disease where cells in the central nervous system play a critical role. To our knowledge, alemtuzumab, fumarates (dimethyl, diroximel, and monomethyl), glatiramer acetates, interferons, mitoxantrone, natalizumab, ocrelizumab, ofatumumab, and teriflunomide are either limited to the periphery or insufficiently studied to confirm direct central nervous system effects in participants with multiple sclerosis. In contrast, cladribine and sphingosine 1-phosphate receptor modulators (fingolimod, ozanimod, ponesimod, and siponimod) are central nervous system-penetrant and could have beneficial direct central nervous system properties.
Keywords: central nervous system; cladribine; fingolimod hydrochloride; multiple sclerosis; ozanimod; ponesimod; siponimod; sphingosine 1 phosphate receptor modulators.
Copyright © 2023 Hartung, Cree, Barnett, Meuth, Bar-Or and Steinman.
Conflict of interest statement
Author H-PH: Personal fees for consulting, serving on steering committees, and speaking from Bayer Healthcare, Biogen, Celgene, GeNeuro, Genzyme, MedImmune, Merck, Novartis, Octapharma, Roche, Sanofi, and Teva. Author BACC: Personal compensation for consulting for Alexion, Atara, Autobahn, Avotres, Biogen, Boston Pharma, EMD Serono, Gossamer Bio, Hexal/Sandoz, Horizon, Immunic AG, Neuron23, Novartis, Sanofi, Siemens, TG Therapeutics, and Therini; and received research support from Genentech. Author MB: Institutional funding from Alexion, Biogen, Bristol Myers Squibb, Merck, Novartis, and Roche; personal compensation for speaking from Biogen and Novartis. Author SGM: Honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Bristol Myers Squibb/Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, Ono Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research, Bundesinstitut für Risikobewertung, Deutsche Forschungsgemeinschaft, Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Research Muenster, German Foundation Neurology, and Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, Ono Pharma, Roche, and Teva. Author AB-O: Received fees for advisory board participation and/or consulting from Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, MedImmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme; and has received grant support to the University of Pennsylvania from Biogen Idec, Merck/EMD Serono, Novartis, and Roche/Genentech. Author LS: Consulting for AbbVie, Atreca, Bristol Myers Squibb, EMD Serono, Novartis, Teva, Pasithea, 180 Life Sciences, and TG Therapeutics, and research support from Atara and Bristol Myers Squibb. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Alemtuzumab, cladribine, dimethyl fumarate, fingolimod, natalizumab, ocrelizumab, ofatumumab, ozanimod, ponesimod, and teriflunomide for the treatment of adult patients with highly active relapsing remitting multiple sclerosis: IQWiG Reports – Commission No. A20-60 [Internet].Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2024 Jan 24. Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2024 Jan 24. PMID: 38386940 Free Books & Documents. Review.
-
Multiple Sclerosis Agents.2022 Sep 7. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2022 Sep 7. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643690 Free Books & Documents. Review.
-
Drugs for multiple sclerosis.Med Lett Drugs Ther. 2021 Mar 22;63(1620):42-48. Med Lett Drugs Ther. 2021. PMID: 33976089 Review. No abstract available.
-
Ozanimod (Zeposia) for multiple sclerosis.Med Lett Drugs Ther. 2020 Aug 24;62(1605):132-134. Med Lett Drugs Ther. 2020. PMID: 32970043 Review. No abstract available.
-
Safety and Monitoring of the Treatment with Disease-Modifying Therapies (DMTs) for Multiple Sclerosis (MS).Curr Rev Clin Exp Pharmacol. 2023;18(1):39-50. doi: 10.2174/2772432817666220412110720. Curr Rev Clin Exp Pharmacol. 2023. PMID: 35418296 Review.
Cited by
-
Digital Pathology Identifies Associations between Tissue Inflammatory Biomarkers and Multiple Sclerosis Outcomes.Cells. 2024 Jun 11;13(12):1020. doi: 10.3390/cells13121020. Cells. 2024. PMID: 38920650 Free PMC article.
-
Profile of Cytokines Associated with SARS-CoV2 Seropositivity in Multiple Sclerosis Patients and Its Persistence over Six Months.J Clin Med. 2025 May 26;14(11):3736. doi: 10.3390/jcm14113736. J Clin Med. 2025. PMID: 40507498 Free PMC article.
-
Dermatological Neoplastic Diseases Complicating Treatment with Monoclonal Antibodies for Multiple Sclerosis.J Clin Med. 2024 Aug 29;13(17):5133. doi: 10.3390/jcm13175133. J Clin Med. 2024. PMID: 39274345 Free PMC article. Review.
-
Sphingosine 1-phosphate receptor modulators in multiple sclerosis treatment: A practical review.Ann Clin Transl Neurol. 2024 Apr;11(4):842-855. doi: 10.1002/acn3.52017. Epub 2024 Feb 16. Ann Clin Transl Neurol. 2024. PMID: 38366285 Free PMC article. Review.
References
-
- Håkansson I. Biomarkers and Disease Activity in Multiple Sclerosis : A Cohort Study on Patients with Clinically Isolated Syndrome and Relapsing Remitting Multiple Sclerosis. Linköping, Sweden: Linköping University Electronic Press; (2019) p. 1–78. Available at: http://liu.diva-portal.org/smash/get/diva2:1358189/PREVIEW01.png. doi: 10.3384/diss.diva-160762 - DOI

