Nanomedicine-Based Drug-Targeting in Breast Cancer: Pharmacokinetics, Clinical Progress, and Challenges
- PMID: 38162753
- PMCID: PMC10753706
- DOI: 10.1021/acsomega.3c07345
Nanomedicine-Based Drug-Targeting in Breast Cancer: Pharmacokinetics, Clinical Progress, and Challenges
Abstract
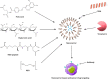
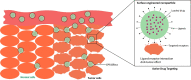
Breast cancer (BC) is a malignant neoplasm that begins in the breast tissue. After skin cancer, BC is the second most common type of cancer in women. At the end of 2040, the number of newly diagnosed BC cases is projected to increase by over 40%, reaching approximately 3 million worldwide annually. The hormonal and chemotherapeutic approaches based on conventional formulations have inappropriate therapeutic effects and suboptimal pharmacokinetic responses with nonspecific targeting actions. To overcome such issues, the use of nanomedicines, including liposomes, nanoparticles, micelles, hybrid nanoparticles, etc., has gained wider attention in the treatment of BC. Smaller dimensional nanomedicine (especially 50-200 nm) exhibited improved in vivo effectiveness, such as better tissue penetration and more effective tumor suppression through enhanced retention and permeation, as well as active targeting of the drug. Additionally, nanotechnology, which further extended and developed theranostic nanomedicine by incorporating diagnostic and imaging agents in one platform, has been applied to BC. Furthermore, hybrid and theranostic nanomedicine has also been explored for gene delivery as anticancer therapeutics in BC. Moreover, the nanocarriers' size, shape, surface charge, chemical compositions, and surface area play an important role in the nanocarriers' stability, cellular absorption, cytotoxicity, cellular uptake, and toxicity. Additionally, nanomedicine clinical translation for managing BC remains a slow process. However, a few cases are being used clinically, and their progress with the current challenges is addressed in this Review. Therefore, this Review extensively discusses recent advancements in nanomedicine and its clinical challenges in BC.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Data Explorer. ECIS - European Cancer Information System. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0 (accessed 2023-09-04).
Publication types
LinkOut - more resources
Full Text Sources

