A review of sources, pathways, and toxic effects of human exposure to benzophenone ultraviolet light filters
- PMID: 38162868
- PMCID: PMC10757257
- DOI: 10.1016/j.eehl.2023.10.001
A review of sources, pathways, and toxic effects of human exposure to benzophenone ultraviolet light filters
Abstract
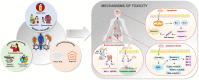
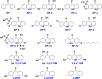
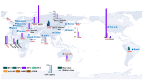
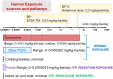
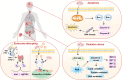
Benzophenone ultraviolet light filters (BPs) are high-production-volume chemicals extensively used in personal care products, leading to widespread human exposure. Given their estrogenic properties, the potential health risks associated with exposure to BPs have become a public health concern. This review aims to summarize sources and pathways of exposure to BPs and associated health risks. Dermal exposure, primarily through the use of sunscreens, constitutes a major pathway for BP exposure. At a recommended application rate, dermal exposure of BP-3 via the application of sunscreens may reach or exceed the suggested reference dose. Other exposure pathways to BPs, such as drinking water, seafood, and packaged foods, contribute minimal to the overall dose. Inhalation is a minor pathway of exposure; however, its contribution cannot be ignored. Human exposure to BPs is an order of magnitude higher in North America than in Asia and Europe. Studies conducted on laboratory animals and cells have consistently demonstrated the toxic effects of BP exposure. BPs are estrogenic and elicit reproductive and developmental toxicities. Furthermore, neurotoxicity, hepatotoxicity, nephrotoxicity, and carcinogenicity have been reported from chronic BP exposure. In addition to animal and cell studies, epidemiological investigations have identified associations between BPs and couples' fecundity and other reproductive disorders, as well as adverse birth outcomes. Further studies are urgently needed to understand the risks posed by BPs on human health.
Keywords: BP-3; Benzophenone ultraviolet light filters; Health effects; Human exposure; Risk assessment.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Safety of benzophenone-type UV filters: A mini review focusing on carcinogenicity, reproductive and developmental toxicity.Chemosphere. 2023 Jun;326:138455. doi: 10.1016/j.chemosphere.2023.138455. Epub 2023 Mar 20. Chemosphere. 2023. PMID: 36944403 Review.
-
Fish consumption is an indicator of exposure to benzophenone derivatives: A probabilistic risk assessment in Taiwanese population.Sci Total Environ. 2022 Mar 15;812:152421. doi: 10.1016/j.scitotenv.2021.152421. Epub 2021 Dec 21. Sci Total Environ. 2022. PMID: 34942259
-
Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review.Environ Int. 2014 Sep;70:143-57. doi: 10.1016/j.envint.2014.05.015. Epub 2014 Jun 14. Environ Int. 2014. PMID: 24934855 Review.
-
Comprehensive Survey of 14 Benzophenone UV Filters in Sunscreen Products Marketed in the United States: Implications for Human Exposure.Environ Sci Technol. 2022 Sep 6;56(17):12473-12482. doi: 10.1021/acs.est.2c03885. Epub 2022 Aug 11. Environ Sci Technol. 2022. PMID: 35951380
-
Urinary concentrations of benzophenone-type ultraviolet radiation filters and couples' fecundity.Am J Epidemiol. 2014 Dec 15;180(12):1168-75. doi: 10.1093/aje/kwu285. Epub 2014 Nov 13. Am J Epidemiol. 2014. PMID: 25395025 Free PMC article.
Cited by
-
Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain.Toxics. 2024 Dec 13;12(12):906. doi: 10.3390/toxics12120906. Toxics. 2024. PMID: 39771121 Free PMC article.
-
Suspect screening of bisphenol A (BPA) structural analogues and functional alternatives in human milk from Canada and South Africa.J Expo Sci Environ Epidemiol. 2025 Jul;35(4):557-566. doi: 10.1038/s41370-025-00782-2. Epub 2025 Jun 2. J Expo Sci Environ Epidemiol. 2025. PMID: 40456860
-
Migration of Chemical Compounds from Packaging Materials into Packaged Foods: Interaction, Mechanism, Assessment, and Regulations.Foods. 2024 Sep 30;13(19):3125. doi: 10.3390/foods13193125. Foods. 2024. PMID: 39410160 Free PMC article. Review.
-
Preliminary Study on the Positive Expression Regulation of Alpha2-Macroglobulin in the Testicular Tissue of Male Mice by Environmental Estrogens.Int J Mol Sci. 2024 Dec 15;25(24):13434. doi: 10.3390/ijms252413434. Int J Mol Sci. 2024. PMID: 39769199 Free PMC article.
-
Association of Phenols, Parabens, and Their Mixture with Maternal Blood Pressure Measurements in the PROTECT Cohort.Environ Health Perspect. 2024 Aug;132(8):87004. doi: 10.1289/EHP14008. Epub 2024 Aug 14. Environ Health Perspect. 2024. PMID: 39140735 Free PMC article.
References
-
- Dupont E., Gomez J., Bilodeau D. Beyond UV radiation: a skin under challenge. Int. J. Cosmet. Sci. 2013;35(3):224–232. - PubMed
-
- Sanchez-Quiles D., Tovar-Sanchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015;83:158–170. - PubMed
-
- Shaath N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010;9(4):464–469. - PubMed
-
- Zhou Y., Cheng F., He D., Zhang Y.N., Qu J., Yang X., Chen J., Peijnenburg W. Effect of UV/chlorine treatment on photophysical and photochemical properties of dissolved organic matter. Water Res. 2021;192 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

