The significance of long non-coding RNAs in the pathogenesis, diagnosis and treatment of inflammatory bowel disease
- PMID: 38163004
- PMCID: PMC10757071
- DOI: 10.1093/pcmedi/pbad031
The significance of long non-coding RNAs in the pathogenesis, diagnosis and treatment of inflammatory bowel disease
Abstract
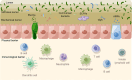
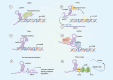
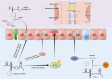
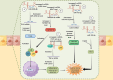
Inflammatory bowel diseases (IBD) are a group of chronic relapsing gastrointestinal inflammatory diseases with significant global incidence. Although the pathomechanism of IBD has been extensively investigated, several aspects of its pathogenesis remain unclear. Long non-coding RNAs (lncRNAs) are transcripts with more than 200 nucleotides in length that have potential protein-coding functions. LncRNAs play important roles in biological processes such as epigenetic modification, transcriptional regulation and post-transcriptional regulation. In this review, we summarize recent advances in research on IBD-related lncRNAs from the perspective of the overall intestinal microenvironment, as well as their potential roles as immune regulators, diagnostic biomarkers and therapeutic targets or agents for IBD.
Keywords: diagnostic biomarker; immune regulator; inflammatory bowel diseases; intestinal barrier; intestinal microenvironment; lncRNAs; therapeutic target.
© The Author(s) 2023. Published by Oxford University Press on behalf of the West China School of Medicine & West China Hospital of Sichuan University.
Conflict of interest statement
All authors declared no conflict of interest. In addition, as an Editorial Board Member of Precision Clinical Medicine, the corresponding author Min Wu was blinded from reviewing or making decisions on this manuscript.
Figures


Similar articles
-
Long non-coding RNAs as pathophysiological regulators, therapeutic targets and novel extracellular vesicle biomarkers for the diagnosis of inflammatory bowel disease.Biomed Pharmacother. 2024 Jul;176:116868. doi: 10.1016/j.biopha.2024.116868. Epub 2024 Jun 7. Biomed Pharmacother. 2024. PMID: 38850647 Review.
-
Biological Function of Long Non-coding RNA (LncRNA) Xist.Front Cell Dev Biol. 2021 Jun 10;9:645647. doi: 10.3389/fcell.2021.645647. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34178980 Free PMC article. Review.
-
Which long noncoding RNAs and circular RNAs contribute to inflammatory bowel disease?Cell Death Dis. 2020 Jun 15;11(6):456. doi: 10.1038/s41419-020-2657-z. Cell Death Dis. 2020. PMID: 32541691 Free PMC article. Review.
-
Exosomes as a New Delivery Vehicle in Inflammatory Bowel Disease.Pharmaceutics. 2021 Oct 9;13(10):1644. doi: 10.3390/pharmaceutics13101644. Pharmaceutics. 2021. PMID: 34683937 Free PMC article. Review.
-
The contribution of long non-coding RNAs in Inflammatory Bowel Diseases.Dig Liver Dis. 2017 Oct;49(10):1067-1072. doi: 10.1016/j.dld.2017.08.003. Epub 2017 Aug 9. Dig Liver Dis. 2017. PMID: 28869157 Review.
Cited by
-
Anti-TNFα in inflammatory bowel disease: from originators to biosimilars.Front Pharmacol. 2024 Jul 24;15:1424606. doi: 10.3389/fphar.2024.1424606. eCollection 2024. Front Pharmacol. 2024. PMID: 39114362 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
