Severe central nervous system demyelination in Sanfilippo disease
- PMID: 38163061
- PMCID: PMC10756675
- DOI: 10.3389/fnmol.2023.1323449
Severe central nervous system demyelination in Sanfilippo disease
Abstract
Introduction: Chronic progressive neuroinflammation is a hallmark of neurological lysosomal storage diseases, including mucopolysaccharidosis III (MPS III or Sanfilippo disease). Since neuroinflammation is linked to white matter tract pathology, we analyzed axonal myelination and white matter density in the mouse model of MPS IIIC HgsnatP304L and post-mortem brain samples of MPS III patients.
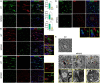
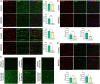
Methods: Brain and spinal cord tissues of human MPS III patients, 6-month-old HgsnatP304L mice and age- and sex-matching wild type mice were analyzed by immunofluorescence to assess levels of myelin-associated proteins, primary and secondary storage materials, and levels of microgliosis. Corpus callosum (CC) region was studied by transmission electron microscopy to analyze axon myelination and morphology of oligodendrocytes and microglia. Mouse brains were analyzed ex vivo by high-filed MRI using Diffusion Basis Spectrum Imaging in Python-Diffusion tensor imaging algorithms.
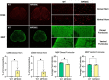
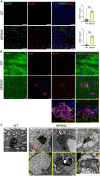
Results: Analyses of CC and spinal cord tissues by immunohistochemistry revealed substantially reduced levels of myelin-associated proteins including Myelin Basic Protein, Myelin Associated Glycoprotein, and Myelin Oligodendrocyte Glycoprotein. Furthermore, ultrastructural analyses revealed disruption of myelin sheath organization and reduced myelin thickness in the brains of MPS IIIC mice and human MPS IIIC patients compared to healthy controls. Oligodendrocytes (OLs) in the CC of MPS IIIC mice were scarce, while examination of the remaining cells revealed numerous enlarged lysosomes containing heparan sulfate, GM3 ganglioside or "zebra bodies" consistent with accumulation of lipids and myelin fragments. In addition, OLs contained swollen mitochondria with largely dissolved cristae, resembling those previously identified in the dysfunctional neurons of MPS IIIC mice. Ex vivo Diffusion Basis Spectrum Imaging revealed compelling signs of demyelination (26% increase in radial diffusivity) and tissue loss (76% increase in hindered diffusivity) in CC of MPS IIIC mice.
Discussion: Our findings demonstrate an important role for white matter injury in the pathophysiology of MPS III. This study also defines specific parameters and brain regions for MRI analysis and suggests that it may become a crucial non-invasive method to evaluate disease progression and therapeutic response.
Keywords: GM3 ganglioside; diffusion basis spectrum imaging; lysosomal storage; mucopolysaccharidosis; myelination; oligodendrocyte.
Copyright © 2023 Taherzadeh, Zhang, Londono, De Leener, Wang, Cooper, Kennedy, Morales, Chen, Lodygensky and Pshezhetsky.
Conflict of interest statement
AVP was a shareholder and received honoraria and research contracts from Phoenix Nest Inc involved in development of therapies for MPS IIID and IIIC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Transplantation of Wild-Type Hematopoietic Stem and Progenitor Cells Improves Disease Phenotypes in a Mucopolysaccharidosis IIIC Mouse Model.Cell Transplant. 2025 Jan-Dec;34:9636897251323966. doi: 10.1177/09636897251323966. Epub 2025 Mar 24. Cell Transplant. 2025. PMID: 40126917 Free PMC article.
-
Heterologous HSPC Transplantation Rescues Neuroinflammation and Ameliorates Peripheral Manifestations in the Mouse Model of Lysosomal Transmembrane Enzyme Deficiency, MPS IIIC.Cells. 2024 May 20;13(10):877. doi: 10.3390/cells13100877. Cells. 2024. PMID: 38786099 Free PMC article.
-
Cathepsin B inhibition blocks amyloidogenesis in the mouse models of neurological lysosomal diseases MPS IIIC and sialidosis.Mol Ther Methods Clin Dev. 2025 Feb 11;33(1):101432. doi: 10.1016/j.omtm.2025.101432. eCollection 2025 Mar 13. Mol Ther Methods Clin Dev. 2025. PMID: 40092638 Free PMC article.
-
Glycosaminoglycans and mucopolysaccharidosis type III.Front Biosci (Landmark Ed). 2016 Jun 1;21(7):1393-409. doi: 10.2741/4463. Front Biosci (Landmark Ed). 2016. PMID: 27100513 Review.
-
Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury.Neuropharmacology. 2016 Nov;110(Pt B):654-659. doi: 10.1016/j.neuropharm.2015.04.029. Epub 2015 May 9. Neuropharmacology. 2016. PMID: 25963414 Review.
Cited by
-
Transplantation of Wild-Type Hematopoietic Stem and Progenitor Cells Improves Disease Phenotypes in a Mucopolysaccharidosis IIIC Mouse Model.Cell Transplant. 2025 Jan-Dec;34:9636897251323966. doi: 10.1177/09636897251323966. Epub 2025 Mar 24. Cell Transplant. 2025. PMID: 40126917 Free PMC article.
-
Heterologous HSPC Transplantation Rescues Neuroinflammation and Ameliorates Peripheral Manifestations in the Mouse Model of Lysosomal Transmembrane Enzyme Deficiency, MPS IIIC.Cells. 2024 May 20;13(10):877. doi: 10.3390/cells13100877. Cells. 2024. PMID: 38786099 Free PMC article.
-
Mucopolysaccharidosis-Plus Syndrome: Is This a Type of Mucopolysaccharidosis or a Separate Kind of Metabolic Disease?Int J Mol Sci. 2024 Sep 4;25(17):9570. doi: 10.3390/ijms25179570. Int J Mol Sci. 2024. PMID: 39273517 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

