LncRNA SNHG8 upregulates MUC5B to induce idiopathic pulmonary fibrosis progression by targeting miR-4701-5p
- PMID: 38163156
- PMCID: PMC10756985
- DOI: 10.1016/j.heliyon.2023.e23233
LncRNA SNHG8 upregulates MUC5B to induce idiopathic pulmonary fibrosis progression by targeting miR-4701-5p
Abstract
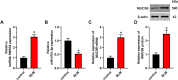
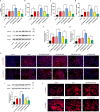
Long noncoding RNAs (lncRNAs) play a critical role in idiopathic pulmonary fibrosis (IPF); however, the underlying molecular mechanisms are unclear. Our study demonstrated that lncRNA small nucleolar RNA host gene 8 (SNHG8) was increased in bleomycin (BLM)-induced A549 cells. LncRNA SNHG8 overexpression further elevated fibrosis-related factors monocyte chemotactic protein 1 (MCP1), CC motif chemokine ligand 18 (CCL18), and α-smooth muscle actin (α-SMA), as well as increased collagen type I alpha-1 chain (COL1A1) and collagen type III alpha-1 chain (COL3A1). Meanwhile, lncRNA SNHG8 knockdown exhibited an opposite role in reducing BLM-induced pulmonary fibrosis. With regard to the mechanism, SNHG8 was then revealed to act as a competing endogenous RNA (ceRNA) for microRNA (miR)-4701-5p in regulating Mucin 5B (MUC5B) expression. Furthermore, the interactions between SNHG8 and miR-4701-5p, between miR-4701-5p and MUC5B, and between SNHG8 and MUC5B on the influence of fibrosis-related indicators were confirmed, respectively. In addition, SNHG8 overexpression enhanced the levels of transforming growth factor (TGF)-β1 and phosphorylation Smad2/3 (p-Smad2/3), which was suppressed by SNHG8 knockdown in BLM-induced A549 cells. Moreover, miR-4701-5p inhibitor-induced elevation of TGF-β1 and p-Smad2/3 was significantly suppressed by SNHG8 knockdown. In conclusion, SNHG8 knockdown attenuated pulmonary fibrosis progression by regulating miR-4701-5p/MUC5B axis, which might be associated with the modulation of TGF-β1/Smad2/3 signaling. These findings reveal that lncRNA SNHG8 may become a potential target for the treatment of IPF.
Keywords: IPF; MUC5B; TGF-β1/Smad2/3 signaling; lncRNA SNHG8; miR-4701-5p.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Moss B.J., Ryter S.W., Rosas I.O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2022;17:515–546. - PubMed
-
- Schafer S.C., Funke-Chambour M., Berezowska S. Idiopathic pulmonary fibrosis-epidemiology, causes, and clinical course. Pathologe. 2020;41:46–51. - PubMed
-
- Drakopanagiotakis F., Wujak L., Wygrecka M., Markart P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018;68–69:404–421. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

