Network pharmacology and experimental evidence: MAPK signaling pathway is involved in the anti-asthma roles of Perilla frutescens leaf
- PMID: 38163225
- PMCID: PMC10755271
- DOI: 10.1016/j.heliyon.2023.e22971
Network pharmacology and experimental evidence: MAPK signaling pathway is involved in the anti-asthma roles of Perilla frutescens leaf
Abstract
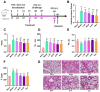
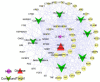
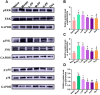
Perilla frutescens (PF) leaf is a traditional Chinese medicine and food with beneficial effects on allergic asthma. We sought to elucidate the active compounds, the targets, and underlying mechanisms of PF leaf in the treatment of allergic asthma by using experimental pharmacology and network pharmacology. An OVA-allergic asthma murine model was constructed to evaluate the effect of PF leaf on allergic asthma. And the network pharmacology and western blotting were performed to evaluate its underlying mechanisms in allergic asthma. PF leaf treatment significantly improved the lung function of OVA model mice and mitigated lung injury by significantly reducing of OVA-specific immunoglobulin E in serum, and interleukin 4, interleukin 5 and tumor necrosis factor alpha in the bronchoalveolar lavage fluid. 50 core targets were screened based on 8 compounds (determined by high performance liquid chromatography) through compound-target- disease network. Furthermore, MAPK signaling pathway was identified as the pathway mediated by PF leaf with the most potential against allergic asthma. And the WB results showed that PF leaf could down-regulate the expression of p-ERK, p-JNK and p-p38, which was highly consistent with the predicted targets and pathway network. In conclusion, this study provides the evidence to support the molecular mechanisms of PF leaf on the treatment of allergic asthma using network pharmacology and in vivo experiments.
Keywords: MAPK pathway; Network pharmacology; OVA-Allergic asthma murine model; Perilla leaves.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Integrated plasma pharmacochemistry and network pharmacology to explore the mechanism of Gerberae Piloselloidis Herba in treatment of allergic asthma.J Ethnopharmacol. 2022 Nov 15;298:115624. doi: 10.1016/j.jep.2022.115624. Epub 2022 Aug 12. J Ethnopharmacol. 2022. PMID: 35970314
-
Integrated systems pharmacology and transcriptomics to dissect the mechanisms of Loki Zupa decoction in the treatment of murine allergic asthma.J Ethnopharmacol. 2022 Aug 10;294:115351. doi: 10.1016/j.jep.2022.115351. Epub 2022 May 6. J Ethnopharmacol. 2022. PMID: 35533913
-
Leaf Extract of Perilla frutescens (L.) Britt Promotes Adipocyte Browning via the p38 MAPK Pathway and PI3K-AKT Pathway.Nutrients. 2023 Mar 20;15(6):1487. doi: 10.3390/nu15061487. Nutrients. 2023. PMID: 36986217 Free PMC article.
-
Metabolomics combined with network pharmacology reveals anti-asthmatic effects of Nepeta bracteata on allergic asthma rats.Chin Herb Med. 2024 Mar 26;16(4):599-611. doi: 10.1016/j.chmed.2024.02.001. eCollection 2024 Oct. Chin Herb Med. 2024. PMID: 39606263 Free PMC article.
-
Revealing the mechanisms of Arisaema cum Bile on allergic asthma with systematic pharmacology approach-experimental validation.Fitoterapia. 2023 Jul;168:105518. doi: 10.1016/j.fitote.2023.105518. Epub 2023 Apr 28. Fitoterapia. 2023. PMID: 37121408
Cited by
-
Comprehensive Review of Perilla frutescens: Chemical Composition, Pharmacological Mechanisms, and Industrial Applications in Food and Health Products.Foods. 2025 Apr 3;14(7):1252. doi: 10.3390/foods14071252. Foods. 2025. PMID: 40238536 Free PMC article. Review.
-
Astragalus membranaceus, Nigella sativa, and Perilla frutescens as Immunomodulators-Molecular Mechanisms and Clinical Effectiveness in Allergic Diseases.Curr Issues Mol Biol. 2024 Aug 17;46(8):9016-9032. doi: 10.3390/cimb46080533. Curr Issues Mol Biol. 2024. PMID: 39194750 Free PMC article. Review.
References
-
- Cevhertas L., Ogulur I., Maurer D.J., Burla D., Ding M., Jansen K., Koch J., Liu C., Ma S., Mitamura Y., Peng Y., Radzikowska U., Rinaldi A.O., Satitsuksanoa P., Globinska A., van de Veen W., Sokolowska M., Baerenfaller K., Gao Y.D., Agache I., Akdis M., Akdis C.A. Advances and recent developments in asthma in 2020. Allergy. 2020;75(12):3124–3146. doi: 10.1111/all.14607. - DOI - PubMed
-
- Xepapadaki P., Bachert C., Finotto S., Jartti T., Konstantinou G.N., Kiefer A., Kowalski M., Lewandowska-Polak A., Lukkarinen H., Roumpedaki E., Sobanska A., Sintobin I., Vuorinen T., Zhang N., Zimmermann T., Papadopoulos N.G. Contribution of repeated infections in asthma persistence from preschool to school age: design and characteristics of the PreDicta cohort. Pediatr. Allergy Immunol. 2018;29(4):383–393. doi: 10.1111/pai.12881. - DOI - PubMed
-
- Liu Changda, Cao Mingzhuo, Yang Nan, Reid‐Adam Jessica, Tversky Jody, Zhan Jixun, Li Xiu‐Min. Time‐dependent dual beneficial modulation of interferon‐γ, interleukin 5, and Treg cytokines in asthma patient peripheral blood mononuclear cells by ganoderic acid B. Phytother Res. 2022;36(3):1231–1240. doi: 10.1002/ptr.7266. - DOI - PubMed
-
- Jayaprakasam B., Yang N., Wen M.C., Wang R., Goldfarb J., Sampson H., Li X.M. Constituents of the anti-asthma herbal formula ASHMI(TM) synergistically inhibit IL-4 and IL-5 secretion by murine Th2 memory cells, and eotaxin by human lung fibroblasts in vitro. J. Integr. Med. 2013;11(3):195–205. doi: 10.3736/jintegrmed2013029. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

