Milestones in understanding transport, sensing, and signaling of the plant nutrient phosphorus
- PMID: 38163641
- PMCID: PMC11062440
- DOI: 10.1093/plcell/koad326
Milestones in understanding transport, sensing, and signaling of the plant nutrient phosphorus
Abstract
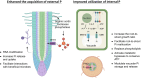
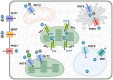
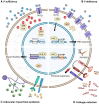
As an essential nutrient element, phosphorus (P) is primarily acquired and translocated as inorganic phosphate (Pi) by plant roots. Pi is often sequestered in the soil and becomes limited for plant growth. Plants have developed a sophisticated array of adaptive responses, termed P starvation responses, to cope with P deficiency by improving its external acquisition and internal utilization. Over the past 2 to 3 decades, remarkable progress has been made toward understanding how plants sense and respond to changing environmental P. This review provides an overview of the molecular mechanisms that regulate or coordinate P starvation responses, emphasizing P transport, sensing, and signaling. We present the major players and regulators responsible for Pi uptake and translocation. We then introduce how P is perceived at the root tip, how systemic P signaling is operated, and the mechanisms by which the intracellular P status is sensed and conveyed. Additionally, the recent exciting findings about the influence of P on plant-microbe interactions are highlighted. Finally, the challenges and prospects concerning the interplay between P and other nutrients and strategies to enhance P utilization efficiency are discussed. Insights obtained from this knowledge may guide future research endeavors in sustainable agriculture.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

