Targeting ferroptosis and ferritinophagy: new targets for cardiovascular diseases
- PMID: 38163663
- PMCID: PMC10758208
- DOI: 10.1631/jzus.B2300097
Targeting ferroptosis and ferritinophagy: new targets for cardiovascular diseases
Abstract
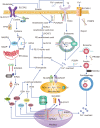
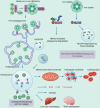
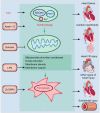
Cardiovascular diseases (CVDs) are a leading factor driving mortality worldwide. Iron, an essential trace mineral, is important in numerous biological processes, and its role in CVDs has raised broad discussion for decades. Iron-mediated cell death, namely ferroptosis, has attracted much attention due to its critical role in cardiomyocyte damage and CVDs. Furthermore, ferritinophagy is the upstream mechanism that induces ferroptosis, and is closely related to CVDs. This review aims to delineate the processes and mechanisms of ferroptosis and ferritinophagy, and the regulatory pathways and molecular targets involved in ferritinophagy, and to determine their roles in CVDs. Furthermore, we discuss the possibility of targeting ferritinophagy-induced ferroptosis modulators for treating CVDs. Collectively, this review offers some new insights into the pathology of CVDs and identifies possible therapeutic targets.
心血管疾病(CVDs)在全球范围内是死亡的主要驱动因素。铁是一种必需的微量元素,在多种生物过程中很重要。几十年来,铁对心血管疾病的作用引起了广泛的讨论。由铁介导的细胞死亡方式,即铁死亡,在心肌细胞损伤和心血管疾病中发挥着重要的作用,因此受到广泛关注。此外,铁自噬是诱导铁死亡的上游机制,与心血管疾病密切相关。本文就铁死亡和铁自噬的过程、机制、铁自噬的调控途径和分子靶点进行综述,并总结其对心血管疾病的作用。此外,我们讨论了针对铁自噬诱导的铁死亡调节剂治疗心血管疾病的可能性。总之,本综述将为心血管疾病的病理学机制提供新的见解,并提供一系列潜在治疗靶点。.
心血管疾病(CVDs)在全球范围内是死亡的主要驱动因素。铁是一种必需的微量元素,在多种生物过程中很重要。几十年来,铁对心血管疾病的作用引起了广泛的讨论。由铁介导的细胞死亡方式,即铁死亡,在心肌细胞损伤和心血管疾病中发挥着重要的作用,因此受到广泛关注。此外,铁自噬是诱导铁死亡的上游机制,与心血管疾病密切相关。本文就铁死亡和铁自噬的过程、机制、铁自噬的调控途径和分子靶点进行综述,并总结其对心血管疾病的作用。此外,我们讨论了针对铁自噬诱导的铁死亡调节剂治疗心血管疾病的可能性。总之,本综述将为心血管疾病的病理学机制提供新的见解,并提供一系列潜在治疗靶点。
Keywords: Cardiovascular disease; Ferritinophagy; Ferroptosis; Iron; Therapeutic target.
Figures
Similar articles
-
Crosstalk of autophagy and ferroptosis in cardiovascular diseases: from pathophysiology to novel therapy.Redox Biol. 2025 Jul;84:103705. doi: 10.1016/j.redox.2025.103705. Epub 2025 May 29. Redox Biol. 2025. PMID: 40450834 Free PMC article. Review.
-
Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications.Biomed Pharmacother. 2021 Sep;141:111872. doi: 10.1016/j.biopha.2021.111872. Epub 2021 Jul 7. Biomed Pharmacother. 2021. PMID: 34246187 Review.
-
Ferritinophagy and ferroptosis in the management of metabolic diseases.Trends Endocrinol Metab. 2021 Jul;32(7):444-462. doi: 10.1016/j.tem.2021.04.010. Epub 2021 May 15. Trends Endocrinol Metab. 2021. PMID: 34006412 Review.
-
Ferritinophagy induced ferroptosis in the management of cancer.Cell Oncol (Dordr). 2024 Feb;47(1):19-35. doi: 10.1007/s13402-023-00858-x. Epub 2023 Sep 15. Cell Oncol (Dordr). 2024. PMID: 37713105 Review.
-
Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases.J Cell Physiol. 2018 Dec;233(12):9179-9190. doi: 10.1002/jcp.26954. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076709 Review.
Cited by
-
The Crosstalk Between Ferritinophagy and Ferroptosis in Ischemic Stroke: Regulatory Mechanisms and Therapeutic Implications.Cell Mol Neurobiol. 2025 Jul 20;45(1):73. doi: 10.1007/s10571-025-01593-7. Cell Mol Neurobiol. 2025. PMID: 40684405 Free PMC article. Review.
-
Decoding glycosylation in cardiovascular diseases: mechanisms, biomarkers, and therapeutic opportunities.Front Pharmacol. 2025 May 19;16:1570158. doi: 10.3389/fphar.2025.1570158. eCollection 2025. Front Pharmacol. 2025. PMID: 40458788 Free PMC article. Review.
References
-
- Aladağ N, Asoğlu R, Ozdemir M, et al. , 2021. Oxidants and antioxidants in myocardial infarction (MI): investigation of ischemia modified albumin, malondialdehyde, superoxide dismutase and catalase in individuals diagnosed with ST elevated myocardial infarction (STEMI) and non-STEMI (NSTEMI). J Med Biochem, 40(3): 286-294. 10.5937/jomb0-28879 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82204389 and 82000454/the National Natural Science Foundation of China
- SBGJ202103079/the Medical Science and Technology Research Project of Henan Province
- LHGJ20230283, LHGJ20190236, LHGJ20190227, LHGJ20190092, LHGJ20200310 and LHGJ20200284/the Henan Medical Science and Technology Joint Building Program
LinkOut - more resources
Full Text Sources

