Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines
- PMID: 38163846
- PMCID: PMC10770148
- DOI: 10.1038/s41422-023-00906-z
Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines
Abstract
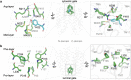
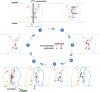
Monoamine neurotransmitters such as serotonin and dopamine are loaded by vesicular monoamine transporter 2 (VMAT2) into synaptic vesicles for storage and subsequent release in neurons. Impaired VMAT2 function underlies various neuropsychiatric diseases. VMAT2 inhibitors reserpine and tetrabenazine are used to treat hypertension, movement disorders associated with Huntington's Disease and Tardive Dyskinesia. Despite its physiological and pharmacological significance, the structural basis underlying VMAT2 substrate recognition and its inhibition by various inhibitors remains unknown. Here we present cryo-EM structures of human apo VMAT2 in addition to states bound to serotonin, tetrabenazine, and reserpine. These structures collectively capture three states, namely the lumen-facing, occluded, and cytosol-facing conformations. Notably, tetrabenazine induces a substantial rearrangement of TM2 and TM7, extending beyond the typical rocker-switch movement. These functionally dynamic snapshots, complemented by biochemical analysis, unveil the essential components responsible for ligand recognition, elucidate the proton-driven exchange cycle, and provide a framework to design improved pharmaceutics targeting VMAT2.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Transport and inhibition mechanisms of human VMAT2.Nature. 2024 Feb;626(7998):427-434. doi: 10.1038/s41586-023-06926-4. Epub 2023 Dec 11. Nature. 2024. PMID: 38081299
-
Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2.Nature. 2023 Nov;623(7989):1086-1092. doi: 10.1038/s41586-023-06727-9. Epub 2023 Nov 1. Nature. 2023. PMID: 37914936
-
Drug inhibition and substrate transport mechanisms of human VMAT2.Nat Commun. 2025 Jan 2;16(1):323. doi: 10.1038/s41467-024-55361-0. Nat Commun. 2025. PMID: 39747030 Free PMC article.
-
Vesicular monoamine transport inhibitors: current uses and future directions.Lancet. 2025 Aug 9;406(10503):650-664. doi: 10.1016/S0140-6736(25)01072-4. Lancet. 2025. PMID: 40783291 Review.
-
VMAT2 inhibitors for the treatment of hyperkinetic movement disorders.Pharmacol Ther. 2020 Aug;212:107580. doi: 10.1016/j.pharmthera.2020.107580. Epub 2020 May 23. Pharmacol Ther. 2020. PMID: 32454050 Review.
Cited by
-
Engineering of a mammalian VMAT2 for cryo-EM analysis results in non-canonical protein folding.Nat Commun. 2024 Aug 2;15(1):6511. doi: 10.1038/s41467-024-50934-5. Nat Commun. 2024. PMID: 39095428 Free PMC article.
-
Monoamine alterations in Alzheimer's disease and their implications in comorbid neuropsychiatric symptoms.Geroscience. 2025 Feb;47(1):457-482. doi: 10.1007/s11357-024-01359-x. Epub 2024 Sep 27. Geroscience. 2025. PMID: 39331291 Free PMC article. Review.
-
Transport and inhibition mechanism for human TauT-mediated taurine uptake.Cell Res. 2025 May;35(5):381-384. doi: 10.1038/s41422-025-01076-w. Epub 2025 Jan 21. Cell Res. 2025. PMID: 39837997 No abstract available.
-
Structural insights into glucose-6-phosphate recognition and hydrolysis by human G6PC1.Proc Natl Acad Sci U S A. 2025 Jan 28;122(4):e2418316122. doi: 10.1073/pnas.2418316122. Epub 2025 Jan 23. Proc Natl Acad Sci U S A. 2025. PMID: 39847333 Free PMC article.
-
Packaging monoamine neurotransmitters.Cell Res. 2024 Mar;34(3):185-186. doi: 10.1038/s41422-023-00922-z. Cell Res. 2024. PMID: 38242944 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- 32171194/National Natural Science Foundation of China (National Science Foundation of China)
- 32371256/National Natural Science Foundation of China (National Science Foundation of China)
- 82225025/National Natural Science Foundation of China (National Science Foundation of China)
- 32071197/National Natural Science Foundation of China (National Science Foundation of China)
- 2022M720805/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

