Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo- and chemo-immunotherapy
- PMID: 38163906
- PMCID: PMC10759660
- DOI: 10.1186/s13046-023-02933-5
Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo- and chemo-immunotherapy
Abstract
Background: About 10% of NSCLCs are mutated in KRAS and impaired in STK11/LKB1, a genetic background associated with poor prognosis, caused by an increase in metastatic burden and resistance to standard therapy. LKB1 is a protein involved in a number of biological processes and is particularly important for its role in the regulation of cell metabolism. LKB1 alterations lead to protein loss that causes mitochondria and metabolic dysfunction that makes cells unable to respond to metabolic stress. Different studies have shown how it is possible to interfere with cancer metabolism using metformin and caloric restriction (CR) and both modify the tumor microenvironment (TME), stimulating the switch from "cold" to "hot". Given the poor therapeutic response of KRASmut/LKB1mut patients, and the role of LKB1 in cell metabolism, we examined whether the addition of metformin and CR enhanced the response to chemo or chemo-immunotherapy in LKB1 impaired tumors.
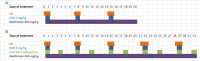
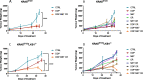
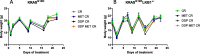
Methods: Mouse cell lines were derived from lung nodules of transgenic mice carrying KRASG12D with either functional LKB1 (KRASG12D/LKB1wt) or mutated LKB1 (KRASG12D/LKB1mut). Once stabilized in vitro, these cell lines were inoculated subcutaneously and intramuscularly into immunocompetent mice. Additionally, a patient-derived xenograft (PDX) model was established by directly implanting tumor fragments from patient into immunocompromised mice. The mice bearing these tumor models were subjected to treatment with chemotherapy or chemo-immunotherapy, both as standalone regimens and in combination with metformin and CR.
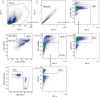
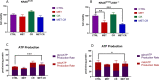
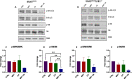
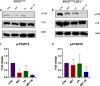
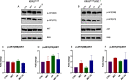
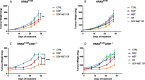
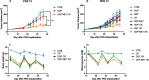
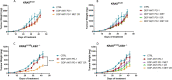
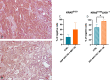
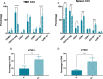
Results: Our preclinical results indicate that in NSCLC KRASmut/LKB1mut tumors, metformin and CR do enhance the response to chemo and chemo-immunotherapy, inducing a metabolic stress condition that these tumors are not able to overcome. Analysis of immune infiltrating cells did not bring to light any strong correlation between the TME immune-modulation and the tumor response to metformin and CR.
Conclusion: Our in vitro and in vivo preliminary studies confirm our hypothesis that the addition of metformin and CR is able to improve the antitumor activity of chemo and chemoimmunotherapy in LKB1 impaired tumors, exploiting their inability to overcome metabolic stress.
Keywords: Caloric restriction; Cancer metabolism; KRAS; LKB1; Metformin; NSCLC.
© 2023. The Author(s).
Conflict of interest statement
M.C. Garassino reports grants and personal fees from Astra Zeneca, Merck; personal fees from BMS, Roche, Daiichi Sankyo, Celgene, GSK, Eli Lilly, Novartis, and personal fees from Regerenon during the conduct of the study.
Figures
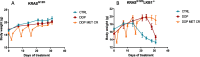
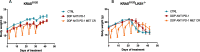
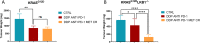
Similar articles
-
Metformin Enhances Cisplatin-Induced Apoptosis and Prevents Resistance to Cisplatin in Co-mutated KRAS/LKB1 NSCLC.J Thorac Oncol. 2018 Nov;13(11):1692-1704. doi: 10.1016/j.jtho.2018.07.102. Epub 2018 Aug 24. J Thorac Oncol. 2018. PMID: 30149143
-
Co-occurring KRAS mutation/LKB1 loss in non-small cell lung cancer cells results in enhanced metabolic activity susceptible to caloric restriction: an in vitro integrated multilevel approach.J Exp Clin Cancer Res. 2018 Dec 4;37(1):302. doi: 10.1186/s13046-018-0954-5. J Exp Clin Cancer Res. 2018. PMID: 30514331 Free PMC article.
-
STK11/LKB1 alterations worsen the poor prognosis of KRAS mutated early-stage non-squamous non-small cell lung carcinoma, results based on the phase 2 IFCT TASTE trial.Lung Cancer. 2024 Apr;190:107508. doi: 10.1016/j.lungcan.2024.107508. Epub 2024 Feb 19. Lung Cancer. 2024. PMID: 38428265 Clinical Trial.
-
Efficacy of immunotherapy in KRAS-mutant non-small-cell lung cancer with comutations.Immunotherapy. 2021 Aug;13(11):941-952. doi: 10.2217/imt-2021-0090. Epub 2021 Jun 11. Immunotherapy. 2021. PMID: 34114474 Review.
-
STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact.Cells. 2021 Nov 11;10(11):3129. doi: 10.3390/cells10113129. Cells. 2021. PMID: 34831355 Free PMC article. Review.
Cited by
-
Metformin promotes anti-tumor immunity in STK11 mutant NSCLC through AXIN1-dependent upregulation of multiple nucleotide metabolites.Oncol Res. 2024 Sep 18;32(10):1637-1648. doi: 10.32604/or.2024.052664. eCollection 2024. Oncol Res. 2024. PMID: 39308524 Free PMC article.
-
Early molecular changes predict cancer cachexia in LKB1-deleted mouse models of NSCLC.Clin Transl Med. 2025 Jul;15(7):e70360. doi: 10.1002/ctm2.70360. Clin Transl Med. 2025. PMID: 40702652 Free PMC article. No abstract available.
-
Current advances in cancer energy metabolism under dietary restriction: a mini review.Med Oncol. 2024 Jul 26;41(9):209. doi: 10.1007/s12032-024-02452-z. Med Oncol. 2024. PMID: 39060824 Review.
-
Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism.Sci Rep. 2024 May 30;14(1):12406. doi: 10.1038/s41598-024-63081-0. Sci Rep. 2024. PMID: 38811809 Free PMC article.
-
LKB1 biology: assessing the therapeutic relevancy of LKB1 inhibitors.Cell Commun Signal. 2024 Jun 6;22(1):310. doi: 10.1186/s12964-024-01689-5. Cell Commun Signal. 2024. PMID: 38844908 Free PMC article. Review.
References
-
- Luo Y-H, Luo L, Wampfler JA, Wang Y, Liu D, Chen Y-M, et al. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: a prospective, observational cohort study. Lancet Oncol. 2019;20:1098–1108. doi: 10.1016/S1470-2045(19)30329-8. - DOI - PMC - PubMed
-
- Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

