Altered larval activation response associated with multidrug resistance in the canine hookworm Ancylostoma caninum
- PMID: 38163962
- PMCID: PMC11007283
- DOI: 10.1017/S0031182023001385
Altered larval activation response associated with multidrug resistance in the canine hookworm Ancylostoma caninum
Abstract
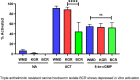
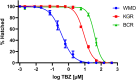
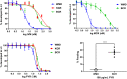
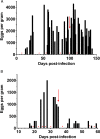
Parasitic gastrointestinal nematodes pose significant health risks to humans, livestock, and companion animals, and their control relies heavily on the use of anthelmintic drugs. Overuse of these drugs has led to the emergence of resistant nematode populations. Herein, a naturally occurring isolate (referred to as BCR) of the dog hookworm, Ancylostoma caninum, that is resistant to 3 major classes of anthelmintics is characterized. Various drug assays were used to determine the resistance of BCR to thiabendazole, ivermectin, moxidectin and pyrantel pamoate. When compared to a drug-susceptible isolate of A. caninum, BCR was shown to be significantly resistant to all 4 of the drugs tested. Multiple single nucleotide polymorphisms have been shown to impart benzimidazole resistance, including the F167Y mutation in the β-tubulin isotype 1 gene, which was confirmed to be present in BCR through molecular analysis. The frequency of the resistant allele in BCR was 76.3% following its first passage in the lab, which represented an increase from approximately 50% in the founding hookworm population. A second, recently described mutation in codon 134 (Q134H) was also detected at lower frequency in the BCR population. Additionally, BCR exhibits an altered larval activation phenotype compared to the susceptible isolate, suggesting differences in the signalling pathways involved in the activation process which may be associated with resistance. Further characterization of this isolate will provide insights into the mechanisms of resistance to macrocyclic lactones and tetrahydropyrimidine anthelmintics.
Keywords: Ancylostoma caninum; benzimidazole; dog hookworm; macrocyclic lactone; multiple anthelmintic drug resistant; pyrantel.
Conflict of interest statement
None.
Figures

Similar articles
-
Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum.Int J Parasitol. 2019 Apr;49(5):397-406. doi: 10.1016/j.ijpara.2018.12.004. Epub 2019 Feb 14. Int J Parasitol. 2019. PMID: 30771359 Free PMC article.
-
Widespread occurrence of benzimidazole resistance single nucleotide polymorphisms in the canine hookworm, Ancylostoma caninum, in Australia.Int J Parasitol. 2025 Mar;55(3-4):173-182. doi: 10.1016/j.ijpara.2024.12.001. Epub 2024 Dec 22. Int J Parasitol. 2025. PMID: 39716589
-
Emergence of Ancylostoma caninum parasites with the benzimidazole resistance F167Y polymorphism in the US dog population.Int J Parasitol Drugs Drug Resist. 2023 Apr;21:131-140. doi: 10.1016/j.ijpddr.2023.01.001. Epub 2023 Mar 14. Int J Parasitol Drugs Drug Resist. 2023. PMID: 36958067 Free PMC article.
-
Compte rendu The canine hookworm Ancylostoma caninum: A novel threat for anthelmintic resistance in Canada.Can Vet J. 2023 Apr;64(4):372-378. Can Vet J. 2023. PMID: 37008647 Free PMC article. Review.
-
Reflecting on the past and fast forwarding to present day anthelmintic resistant Ancylostoma caninum-A critical issue we neglected to forecast.Int J Parasitol Drugs Drug Resist. 2023 Aug;22:36-43. doi: 10.1016/j.ijpddr.2023.04.003. Epub 2023 May 10. Int J Parasitol Drugs Drug Resist. 2023. PMID: 37229949 Free PMC article. Review.
Cited by
-
Anthelmintic Resistance in Ancylostoma caninum: A Comprehensive Review.Vet Med Sci. 2025 Jul;11(4):e70434. doi: 10.1002/vms3.70434. Vet Med Sci. 2025. PMID: 40434926 Free PMC article. Review.
-
Successful Treatment of Cutaneous Larva Migrans With Combined Albendazole and Ivermectin Therapy: A Report of Two Cases From Sudan.Cureus. 2024 Jul 16;16(7):e64665. doi: 10.7759/cureus.64665. eCollection 2024 Jul. Cureus. 2024. PMID: 39021742 Free PMC article.
-
Immunity mediates host specificity in the human hookworm Ancylostoma ceylanicum.Parasitology. 2024 Jan;151(1):102-107. doi: 10.1017/S0031182023001208. Epub 2023 Nov 29. Parasitology. 2024. PMID: 38018393 Free PMC article.
-
Benzimidazole Resistance-Associated Mutations in the β-tubulin Gene of Hookworms: A Systematic Review.Parasitol Res. 2024 Dec 9;123(12):405. doi: 10.1007/s00436-024-08432-6. Parasitol Res. 2024. PMID: 39652258
-
Efficacy of Simparica Trio® against induced infections of Ancylostoma braziliense and Ancylostoma ceylanicum in dogs.Parasit Vectors. 2025 Apr 28;18(1):159. doi: 10.1186/s13071-025-06758-3. Parasit Vectors. 2025. PMID: 40296062 Free PMC article.
References
-
- Albonico M, Wright V and Bickle Q (2004) Molecular analysis of the ß-tubilin gene of human hookworms as a basis for possible benzimidazole resistance on Pemba Island. Molecular and Biochemical Parasitology 134, 1–4. - PubMed
-
- American Veterinary Medical Association (2022) Pet population. In U.S. pet Ownership & Demographics Sourcebook. Schaumburg, IL: Veterinary Economics Division, American Veterinary Medical Association, p.3.
-
- Avramenko RW, Redman EM, Melville L, Bartley Y, Wit J, Queiroz C, Bartley DJ and Gilleard JS (2019) Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. International Journal for Parasitology 49, 13–26. 10.1016/j.ijpara.2018.10.005 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

