Favorable Virological Outcome, Characteristics of Injection Site Reactions, Decrease in Renal Function Biomarkers in Asian People with HIV Receiving Long-Acting Cabotegravir Plus Rilpivirine
- PMID: 38164081
- PMCID: PMC11040190
- DOI: 10.1089/AID.2023.0108
Favorable Virological Outcome, Characteristics of Injection Site Reactions, Decrease in Renal Function Biomarkers in Asian People with HIV Receiving Long-Acting Cabotegravir Plus Rilpivirine
Abstract
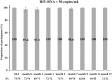
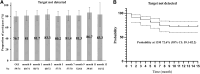
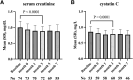
Long-acting cabotegravir plus rilpivirine has revolutionized the concept of antiretroviral therapy, but as the causes of virological failure and satisfaction can depend on patient background, real-world data are needed. In this single-center study, we reviewed clinical records of people with HIV (PWH) who received injectable cabotegravir plus rilpivirine between June 2022 and January 2023. We assessed virological and safety outcomes, including injection site reactions (ISRs) and changes in serum creatinine and cystatin C. Seventy-four patients were included. There were no virological failures. Approximately 80% of individuals achieved HIV-RNA undetectable in all visits up to 14 months (median 13 months) after switching. Pain upon injection was significantly more common at the rilpivirine injection site, while delayed pain was significantly more common at the cabotegravir injection site. The serum creatinine (mean difference -0.12 mg/dL, p < .0001) and the cystatin C (mean difference -0.077 mg/dL, p < .0001) decreased significantly after switching, and in multivariable regression analysis, baseline characteristics did not affect the decrease in these renal function markers. Long-acting cabotegravir plus rilpivirine showed excellent antiviral efficacy and safety in PWH in Japan. ISRs were characterized differently at the cabotegravir and rilpivirine injection sites. Although cystatin C showed decrease after the regimen switch, further confirmation is needed whether cabotegravir plus rilpivirine can improve renal function.
Keywords: HIV; antiretroviral therapy; cabotegravir plus rilpivirine; injection site reaction; long-acting drug.
Conflict of interest statement
E.A. received honorarium from ViiV Healthcare (Brentford, London United Kingdom).
Figures
Similar articles
-
Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study.Lancet HIV. 2021 Nov;8(11):e668-e678. doi: 10.1016/S2352-3018(21)00184-3. Epub 2021 Oct 14. Lancet HIV. 2021. PMID: 34656207 Clinical Trial.
-
Efficacy, safety, and tolerability of switching to long-acting cabotegravir plus rilpivirine versus continuing fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): a randomised, open-label, phase 3b, non-inferiority trial.Lancet HIV. 2023 Sep;10(9):e566-e577. doi: 10.1016/S2352-3018(23)00136-4. Epub 2023 Aug 8. Lancet HIV. 2023. PMID: 37567205 Clinical Trial.
-
Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study.Lancet. 2021 Dec 19;396(10267):1994-2005. doi: 10.1016/S0140-6736(20)32666-0. Epub 2020 Dec 9. Lancet. 2021. PMID: 33308425 Clinical Trial.
-
Cabotegravir/Rilpivirine: the last FDA-approved drug to treat HIV.Expert Rev Anti Infect Ther. 2022 Aug;20(8):1135-1147. doi: 10.1080/14787210.2022.2081153. Epub 2022 Jun 13. Expert Rev Anti Infect Ther. 2022. PMID: 35596583 Review.
-
Question and answers on long-acting therapy with cabotegravir and rilpivirine in people with HIV.J Antimicrob Chemother. 2025 Mar 3;80(3):610-623. doi: 10.1093/jac/dkaf025. J Antimicrob Chemother. 2025. PMID: 39888371 Review.
Cited by
-
Long-Acting Cabotegravir and Rilpivirine Treatment for HIV in Patients With Kidney Failure: Two Cases of Successful Transition to Long-Acting Injectable HIV Therapy.Kidney Med. 2025 Jun 26;7(9):101060. doi: 10.1016/j.xkme.2025.101060. eCollection 2025 Sep. Kidney Med. 2025. PMID: 40837247 Free PMC article.
-
Brief Report: Switching to Long-Acting CAB/RPV: Data From an Italian Monocentric Cohort.J Acquir Immune Defic Syndr. 2024 Dec 1;97(4):e1-e5. doi: 10.1097/QAI.0000000000003501. J Acquir Immune Defic Syndr. 2024. PMID: 40778517 Free PMC article.
References
-
- Overton ET, Richmond G, Rizzardini G, et al. . Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: A randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021;396(10267):1994–2005; doi: 10.1016/s0140-6736(20)32666-0 - DOI - PubMed
-
- Overton ET, Richmond G, Rizzardini G, et al. . Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection: 152-Week results from ATLAS-2M, a randomized, open-label, Phase 3b, noninferiority study. Clin Infect Dis 2023; In Press; doi: 10.1093/cid/ciad020 - DOI - PMC - PubMed
-
- Orkin C, Bernal Morell E, Tan DHS, et al. . Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: Week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021;8(11):e668–e678; doi: 10.1016/s2352-3018(21)00184-3 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
