Clofazimine pharmacokinetics in HIV-infected adults with diarrhea: Implications of diarrheal disease on absorption of orally administered therapeutics
- PMID: 38164114
- PMCID: PMC10941540
- DOI: 10.1002/psp4.13092
Clofazimine pharmacokinetics in HIV-infected adults with diarrhea: Implications of diarrheal disease on absorption of orally administered therapeutics
Abstract
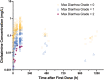
Oral drug absorption kinetics are usually established in populations with a properly functioning gastrointestinal tract. However, many diseases and therapeutics can alter gastrointestinal physiology and cause diarrhea. The extent of diarrhea-associated impact on drug pharmacokinetics has not been quantitatively described. To address this knowledge gap, we used a population pharmacokinetic modeling approach with data collected in a phase IIa study of matched human immunodeficiency virus (HIV)-infected adults with/without cryptosporidiosis and diarrhea to examine diarrhea-associated impact on oral clofazimine pharmacokinetics. A population pharmacokinetic model was developed with 428 plasma samples from 23 HIV-infected adults with/without Cryptosporidium infection using nonlinear mixed-effects modeling. Covariates describing cryptosporidiosis-associated diarrhea severity (e.g., number of diarrhea episodes, diarrhea grade) or HIV infection (e.g., viral load, CD4+ T cell count) were evaluated. A two-compartment model with lag time and first-order absorption and elimination best fit the data. Maximum diarrhea grade over the study duration was found to be associated with a more than sixfold reduction in clofazimine bioavailability. Apparent clofazimine clearance, intercompartmental clearance, central volume of distribution, and peripheral volume of distribution were 3.71 L/h, 18.2 L/h (interindividual variability [IIV] 45.0%), 473 L (IIV 3.46%), and 3434 L, respectively. The absorption rate constant was 0.625 h-1 (IIV 149%) and absorption lag time was 1.83 h. In conclusion, the maximum diarrhea grade observed for the duration of oral clofazimine administration was associated with a significant reduction in clofazimine bioavailability. Our results highlight the importance of studying disease impacts on oral therapeutic pharmacokinetics to inform dose optimization and maximize the chance of treatment success.
© 2023 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

