Two birds with one stone: human SIRPα nanobodies for functional modulation and in vivo imaging of myeloid cells
- PMID: 38164132
- PMCID: PMC10757926
- DOI: 10.3389/fimmu.2023.1264179
Two birds with one stone: human SIRPα nanobodies for functional modulation and in vivo imaging of myeloid cells
Abstract
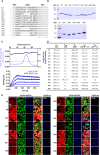
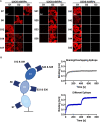
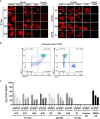
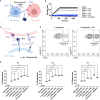
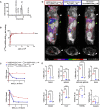
Signal-regulatory protein α (SIRPα) expressed by myeloid cells is of particular interest for therapeutic strategies targeting the interaction between SIRPα and the "don't eat me" ligand CD47 and as a marker to monitor macrophage infiltration into tumor lesions. To address both approaches, we developed a set of novel human SIRPα (hSIRPα)-specific nanobodies (Nbs). We identified high-affinity Nbs targeting the hSIRPα/hCD47 interface, thereby enhancing antibody-dependent cellular phagocytosis. For non-invasive in vivo imaging, we chose S36 Nb as a non-modulating binder. By quantitative positron emission tomography in novel hSIRPα/hCD47 knock-in mice, we demonstrated the applicability of 64Cu-hSIRPα-S36 Nb to visualize tumor infiltration of myeloid cells. We envision that the hSIRPα-Nbs presented in this study have potential as versatile theranostic probes, including novel myeloid-specific checkpoint inhibitors for combinatorial treatment approaches and for in vivo stratification and monitoring of individual responses during cancer immunotherapies.
Keywords: PET imaging tracer; SIRPalpha; immune checkpoint inhibitor (ICI); myeloid cells; nanobodies (Nbs); theranostics.
Copyright © 2023 Wagner, Blaess, Leske, Frecot, Gramlich, Traenkle, Kaiser, Seyfried, Maier, Rezza, Sônego, Thiam, Pezzana, Zeck, Gouttefangeas, Scholz, Nueske, Maurer, Kneilling, Pichler, Sonanini and Rothbauer.
Conflict of interest statement
DSo, MK, BP, TW, BT, PK, and UR are named as inventors on a patent application claiming the use of the described nanobodies for diagnosis and therapeutics filed by the NMI Natural and Medical Sciences Institute and the University of Tübingen. AR, FS, and KT are employees of the company genOway. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

