Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation
- PMID: 38164139
- PMCID: PMC10750202
- DOI: 10.7150/thno.89066
Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation
Abstract
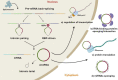
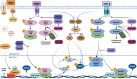
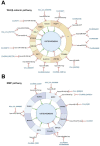
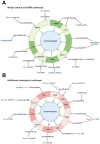
Human osteogenic differentiation is a complex and well-orchestrated process which involves a plethora of molecular players and cellular processes. A growing number of studies have underlined that circular RNAs (circRNAs) play an important regulatory role during human osteogenic differentiation. CircRNAs are single-stranded, covalently closed non-coding RNA molecules that are acquiring increased attention as epigenetic regulators of gene expression. Given their intrinsic high conformational stability, abundance, and specificity, circRNAs can undertake various biological activities in order to regulate multiple cellular processes, including osteogenic differentiation. The most recent evidence indicates that circRNAs control human osteogenesis by preventing the inhibitory activity of miRNAs on their downstream target genes, using a competitive endogenous RNA mechanism. The aim of this review is to draw attention to the currently known regulatory mechanisms of circRNAs during human osteogenic differentiation. Specifically, we provide an understanding of recent advances in research conducted on various human mesenchymal stem cell types that underlined the importance of circRNAs in regulating osteogenesis. A comprehensive understanding of the underlying regulatory mechanisms of circRNA in osteogenesis will improve knowledge on the molecular processes of bone growth, resulting in the potential development of novel preclinical and clinical studies and the discovery of novel diagnostic and therapeutic tools for bone disorders.
Keywords: circRNA; circular RNA; crosstalk; mesenchymal stem cell; miRNA; microRNA; osteogenesis; osteogenic differentiation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

