Bile acid-containing lipid nanoparticles enhance extrahepatic mRNA delivery
- PMID: 38164140
- PMCID: PMC10750194
- DOI: 10.7150/thno.89913
Bile acid-containing lipid nanoparticles enhance extrahepatic mRNA delivery
Abstract
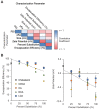
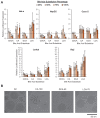
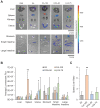
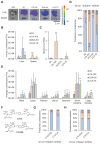
Lipid nanoparticles (LNPs) have emerged as a viable, clinically-validated platform for the delivery of mRNA therapeutics. LNPs have been utilized as mRNA delivery systems for applications including vaccines, gene therapy, and cancer immunotherapy. However, LNPs, which are typically composed of ionizable lipids, cholesterol, helper lipids, and lipid-anchored polyethylene glycol, often traffic to the liver which limits the therapeutic potential of the platform. Several approaches have been proposed to resolve this tropism such as post-synthesis surface modification or the addition of synthetic cationic lipids. Methods: Here, we present a strategy for achieving extrahepatic delivery of mRNA involving the incorporation of bile acids, a naturally-occurring class of cholesterol analogs, during LNP synthesis. We synthesized a series of bile acid-containing C14-4 LNPs by replacing cholesterol with bile acids (cholic acid, chenodeoxycholic acid, deoxycholic acid, or lithocholic acid) at various ratios. Results: Bile acid-containing LNPs (BA-LNPs) were able to reduce delivery to liver cells in vitro and improve delivery in a variety of other cell types, including T cells, B cells, and epithelial cells. Our subsequent in vivo screening of selected LNP candidates injected intraperitoneally or intravenously identified a highly spleen tropic BA-LNP: CA-100, a four-component LNP containing cholic acid and no cholesterol. These screens also identified BA-LNP candidates demonstrating promise for other mRNA therapeutic applications such as for gastrointestinal or immune cell delivery. We further found that the substitution of cholic acid for cholesterol in an LNP formulation utilizing a different ionizable lipid, C12-200, also shifted mRNA delivery from the liver to the spleen, suggesting that this cholic acid replacement strategy may be generalizable. Conclusion: These results demonstrate the potential of a four-component BA-LNP formulation, CA-100, for extrahepatic mRNA delivery that could potentially be utilized for a range of therapeutic and vaccine applications.
Keywords: extrahepatic delivery; lipid nanoparticles; mRNA.
© The author(s).
Conflict of interest statement
Competing Interests: SKP, MMB, and MJM are inventors on patents related to this work filed by the Trustees of the University of Pennsylvania.
Figures

References
-
- Verma M, Ozer I, Xie W, Gallagher R, Teixeira A, Choy M. The landscape for lipid-nanoparticle-based genomic medicines. Nat Rev Drug Discov. 2023;22:349–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

