Functionally Distinct Circuits Are Linked by Heterocellular Electrical Synapses in the Thalamic Reticular Nucleus
- PMID: 38164593
- PMCID: PMC10849028
- DOI: 10.1523/ENEURO.0269-23.2023
Functionally Distinct Circuits Are Linked by Heterocellular Electrical Synapses in the Thalamic Reticular Nucleus
Abstract
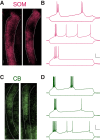
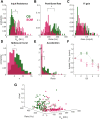
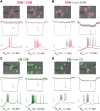
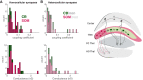
The thalamic reticular nucleus (TRN) inhibits sensory thalamocortical relay neurons and is a key regulator of sensory attention as well as sleep and wake states. Recent developments have identified two distinct genetic subtypes of TRN neurons, calbindin-expressing (CB) and somatostatin-expressing (SOM) neurons. These subtypes differ in localization within the TRN, electrophysiological properties, and importantly, targeting of thalamocortical relay channels. CB neurons send inhibition to and receive excitation from first-order thalamic relay nuclei, while SOM neurons send inhibition to and receive excitation from higher-order thalamic areas. These differences create distinct channels of information flow. It is unknown whether TRN neurons form electrical synapses between SOM and CB neurons and consequently bridge first-order and higher-order thalamic channels. Here, we use GFP reporter mice to label and record from CB-expressing and SOM-expressing TRN neurons. We confirm that GFP expression properly differentiates TRN subtypes based on electrophysiological differences, and we identified electrical synapses between pairs of neurons with and without common GFP expression for both CB and SOM types. That is, electrical synapses link both within and across subtypes of neurons in the TRN, forming either homocellular or heterocellular synapses. Therefore, we conclude that electrical synapses within the TRN provide a substrate for functionally linking thalamocortical first-order and higher-order channels within the TRN.
Keywords: electrical synapse; gap junction; thalamocortical; thalamus.
Copyright © 2024 Vaughn et al.
Figures
Similar articles
-
A mixed electrical and chemical synapse in the thalamic reticular nucleus.J Neurophysiol. 2024 Dec 1;132(6):1955-1963. doi: 10.1152/jn.00339.2024. Epub 2024 Oct 30. J Neurophysiol. 2024. PMID: 39475494
-
Two dynamically distinct circuits drive inhibition in the sensory thalamus.Nature. 2020 Jul;583(7818):813-818. doi: 10.1038/s41586-020-2512-5. Epub 2020 Jul 22. Nature. 2020. PMID: 32699410 Free PMC article.
-
Electrical synapses between inhibitory neurons shape the responses of principal neurons to transient inputs in the thalamus: a modeling study.Sci Rep. 2018 May 17;8(1):7763. doi: 10.1038/s41598-018-25956-x. Sci Rep. 2018. PMID: 29773817 Free PMC article.
-
Bursts modify electrical synaptic strength.Brain Res. 2012 Dec 3;1487:140-9. doi: 10.1016/j.brainres.2012.05.061. Epub 2012 Jul 5. Brain Res. 2012. PMID: 22771703 Free PMC article. Review.
-
Thalamic reticular nucleus in the thalamocortical loop.Neurosci Res. 2020 Jul;156:32-40. doi: 10.1016/j.neures.2019.12.004. Epub 2019 Dec 5. Neurosci Res. 2020. PMID: 31812650 Review.
Cited by
-
Dopamine receptors D1, D2, and D4 modulate electrical synapses and excitability in the thalamic reticular nucleus.J Neurophysiol. 2025 Feb 1;133(2):374-387. doi: 10.1152/jn.00260.2024. Epub 2024 Dec 20. J Neurophysiol. 2025. PMID: 39706150 Free PMC article.
-
Cell type-specific inhibitory modulation of sound processing in the auditory thalamus.bioRxiv [Preprint]. 2025 Mar 9:2024.06.29.601250. doi: 10.1101/2024.06.29.601250. bioRxiv. 2025. PMID: 38979223 Free PMC article. Preprint.
-
Local activation of CB1 receptors by synthetic and endogenous cannabinoids dampens burst firing mode of reticular thalamic nucleus neurons in rats under ketamine anesthesia.Exp Brain Res. 2024 Sep;242(9):2137-2157. doi: 10.1007/s00221-024-06889-6. Epub 2024 Jul 9. Exp Brain Res. 2024. PMID: 38980339
References
-
- Aizenberg M, Martinez SR, Pham T, Rao W, Haas J, Geffen MN (2019) A novel mechanism for amplification of sensory responses by the amygdala–TRN projections. bioRxiv:623868.
-
- Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J, Erlij D (2006) Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience 143:477–486. 10.1016/j.neuroscience.2006.08.033 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
