Expression of Ceramide Synthases in Mice and Their Roles in Regulating Acyl-Chain Sphingolipids: A Framework for Baseline Levels and Future Implications in Aging and Disease
- PMID: 38164625
- PMCID: PMC10877707
- DOI: 10.1124/molpharm.123.000788
Expression of Ceramide Synthases in Mice and Their Roles in Regulating Acyl-Chain Sphingolipids: A Framework for Baseline Levels and Future Implications in Aging and Disease
Abstract
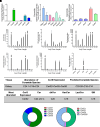
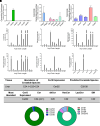
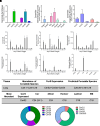
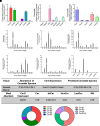
Sphingolipids are an important class of lipids present in all eukaryotic cells that regulate critical cellular processes. Disturbances in sphingolipid homeostasis have been linked to several diseases in humans. Ceramides are central in sphingolipid metabolism and are largely synthesized by six ceramide synthase (CerS) isoforms (CerS1-6), each with a preference for different fatty acyl chain lengths. Although the tissue distribution of CerS mRNA expression in humans and the roles of CerS isoforms in synthesizing ceramides with different acyl chain lengths are known, it is unknown how CerS expression dictates ceramides and downstream metabolites within tissues. In this study, we analyzed sphingolipid levels and CerS mRNA expression in 3-month-old C57BL/6J mouse brain, heart, kidney, liver, lung, and skeletal muscle. The results showed that CerS expression and sphingolipid species abundance varied by tissue and that CerS expression was a predictor of ceramide species within tissues. Interestingly, although CerS expression was not predictive of complex sphingolipid species within all tissues, composite scores for CerSs contributions to total sphingolipids measured in each tissue correlated to CerS expression. Lastly, we determined that the most abundant ceramide species in mouse tissues aligned with CerS mRNA expression in corresponding human tissues (based on chain length preference), suggesting that mice are relevant preclinical models for ceramide and sphingolipid research. SIGNIFICANCE STATEMENT: The current study demonstrates that ceramide synthase (CerS) expression in specific tissues correlates not only with ceramide species but contributes to the generation of complex sphingolipids as well. As many of the CerSs and/or specific ceramide species have been implicated in disease, these studies suggest the potential for CerSs as therapeutic targets and the use of sphingolipid species as diagnostics in specific tissues.
U.S. Government work not protected by U.S. copyright.
Figures


References
-
- Ben-David O, Futerman AH (2010) The role of the ceramide acyl chain length in neurodegeneration: involvement of ceramide synthases. Neuromolecular Med 12:341–350. - PubMed
-
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv Exp Med Biol 688:46–59. - PubMed
-
- Cai XF, Tao Z, Yan ZQ, Yang SL, Gong Y (2003) Molecular cloning, characterisation and tissue-specific expression of human LAG3, a member of the novel Lag1 protein family. DNA Seq 14:79–86. - PubMed
-
- Choi Y, Kim M, Kim SJ, Yoo HJ, Kim SH, Park HS (2020) Metabolic shift favoring C18:0 ceramide accumulation in obese asthma. Allergy 75:2858–2866. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
