The consequence of ATP synthase dimer angle on mitochondrial morphology studied by cryo-electron tomography
- PMID: 38164968
- PMCID: PMC10903453
- DOI: 10.1042/BCJ20230450
The consequence of ATP synthase dimer angle on mitochondrial morphology studied by cryo-electron tomography
Abstract
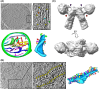
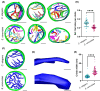
Mitochondrial ATP synthases form rows of dimers, which induce membrane curvature to give cristae their characteristic lamellar or tubular morphology. The angle formed between the central stalks of ATP synthase dimers varies between species. Using cryo-electron tomography and sub-tomogram averaging, we determined the structure of the ATP synthase dimer from the nematode worm C. elegans and show that the dimer angle differs from previously determined structures. The consequences of this species-specific difference at the dimer interface were investigated by comparing C. elegans and S. cerevisiae mitochondrial morphology. We reveal that C. elegans has a larger ATP synthase dimer angle with more lamellar (flatter) cristae when compared to yeast. The underlying cause of this difference was investigated by generating an atomic model of the C. elegans ATP synthase dimer by homology modelling. A comparison of our C. elegans model to an existing S. cerevisiae structure reveals the presence of extensions and rearrangements in C. elegans subunits associated with maintaining the dimer interface. We speculate that increasing dimer angles could provide an advantage for species that inhabit variable-oxygen environments by forming flatter more energetically efficient cristae.
Keywords: alphafold; atp synthase; cryo-electron microscopy; mitochondria; sub-tomogram averaging.
Copyright 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

