Efgartigimod in generalized myasthenia gravis: A real-life experience at a national reference center
- PMID: 38164996
- PMCID: PMC11236067
- DOI: 10.1111/ene.16189
Efgartigimod in generalized myasthenia gravis: A real-life experience at a national reference center
Abstract
Background and purpose: Inhibition of the neonatal Fc receptor (FcRn) for IgG is a promising new therapeutic strategy for antibody-mediated disorders. We report our real-life experience with efgartigimod (EFG) in 19 patients with generalized myasthenia gravis (gMG) along a clinical follow-up of 14 months.
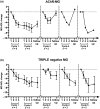
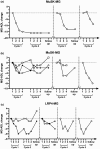
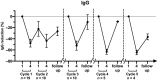
Methods: EFG was administered according to the GENERATIVE protocol (consisting of a Fixed period of two treatment cycles [given 1 month apart] of four infusions at weekly intervals, followed by a Flexible period of re-cycling in case of worsening). Eight patients were positive for acetylcholine receptor antibody, four for muscle-specific tyrosine kinase antibody, and two for lipoprotein-related protein 4 antibody, and five were classified as triple negative. Efficacy of EFG was assessed by the Myasthenia Gravis Activities of Daily Living, Myasthenia Gravis Composite, and Quantitative Myasthenia Gravis scales.
Results: Fifty-three percent of patients needed three treatment cycles, 26% needed four, and 21% needed five along the 14-month clinical follow-up. Meaningful improvement was observed at the end of each cycle with the clinical scores adopted. EFG had a dramatic effect on disease course, as during the year before treatment eight of 19 patients (42%) were hospitalized, and 15 of 19 (79%) needed treatment with plasma exchange or immunoglobulins; three of 19 (16%) were admitted to the intensive care unit. During EFG, none of the patients was hospitalized and only one patient required plasma exchange and intravenous immunoglobulins. No major side effects or infusion-related reactions occurred.
Conclusions: We observed that EFG was safe and modified significantly the course of the disease along a 14-month follow-up. Our experience strengthens the role of FcRn inhibition as an effective new tool for long-term treatment of gMG.
Keywords: MuSK; acetylcholine receptor; efgartigimod; myasthenia gravis; neonatal Fc receptor.
© 2023 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
F.V. has received funding for consulting and speaking from Alexion Pharmaceuticals and argenx. L.M. has received funding for travel, meeting attendance, and advisory board participation from Sanofi Genzyme, Roche, Biogen, Amicus Therapeutics, Alexion, Janssen, Lupin, and argenx. S.B. has received funding for travel, meeting attendance, and advisory board participation from Sanofi Genzyme, Biogen, Alexion, and Roche. R.M. has received funding for travel, meeting attendance and advisory board participation from Alexion, argenx, BioMarin, Catalyst, Sanofi Genzyme, Regeneron, and UCB. C.A. has received funding for travel, meeting attendance, and advisory board participation from Alexion, Momenta, Sanofi, argenx, and UCB. A.P. is an employee of argenx. None of the other authors has any conflict of interest to disclose.
Figures


References
-
- Huijbers MG, Marx A, Plomp JJ, LePanse R, Phillips WD. Advances in understanding of disease mechanisms of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21:163‐175. - PubMed
-
- Punga AR, Maddison P, Heckmann JM, Guptill JT, Evoli A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular disorders. Lancet Neurol. 2022;21:176‐188. - PubMed
-
- Verschuuren JJGN, Palace J, Murai H, Tannemaat MR, Kaminski HJ, Bril V. Advances and ongoing research in the treatment of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21:189‐202. - PubMed
-
- Baggi F, Andreetta F, Maggi L, et al. Complete stable remission and autoantibody specificity in myasthenia gravis. Neurology. 2013;80:188‐195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

