Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation
- PMID: 38165034
- PMCID: PMC10760960
- DOI: 10.1172/JCI173564
Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation
Abstract
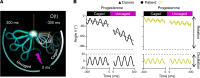
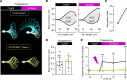
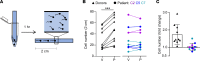
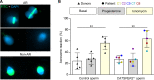
The infertility of many couples rests on an enigmatic dysfunction of the man's sperm. To gain insight into the underlying pathomechanisms, we assessed the function of the sperm-specific multisubunit CatSper-channel complex in the sperm of almost 2,300 men undergoing a fertility workup, using a simple motility-based test. We identified a group of men with normal semen parameters but defective CatSper function. These men or couples failed to conceive naturally and upon medically assisted reproduction via intrauterine insemination and in vitro fertilization. Intracytoplasmic sperm injection (ICSI) was, ultimately, required to conceive a child. We revealed that the defective CatSper function was caused by variations in CATSPER genes. Moreover, we unveiled that CatSper-deficient human sperm were unable to undergo hyperactive motility and, therefore, failed to penetrate the egg coat. Thus, our study provides the experimental evidence that sperm hyperactivation is required for human fertilization, explaining the infertility of CatSper-deficient men and the need of ICSI for medically assisted reproduction. Finally, our study also revealed that defective CatSper function and ensuing failure to hyperactivate represents the most common cause of unexplained male infertility known thus far and that this sperm channelopathy can readily be diagnosed, enabling future evidence-based treatment of affected couples.
Keywords: Calcium channels; Cell Biology; Fertility; Reproductive Biology.
Figures