Newly identified roles for PIEZO1 mechanosensor in controlling normal megakaryocyte development and in primary myelofibrosis
- PMID: 38165047
- PMCID: PMC10922533
- DOI: 10.1002/ajh.27184
Newly identified roles for PIEZO1 mechanosensor in controlling normal megakaryocyte development and in primary myelofibrosis
Abstract
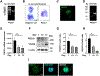
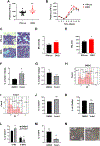
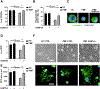
Mechanisms through which mature megakaryocytes (Mks) and their progenitors sense the bone marrow extracellular matrix to promote lineage differentiation in health and disease are still partially understood. We found PIEZO1, a mechanosensitive cation channel, to be expressed in mouse and human Mks. Human mutations in PIEZO1 have been described to be associated with blood cell disorders. Yet, a role for PIEZO1 in megakaryopoiesis and proplatelet formation has never been investigated. Here, we show that activation of PIEZO1 increases the number of immature Mks in mice, while the number of mature Mks and Mk ploidy level are reduced. Piezo1/2 knockout mice show an increase in Mk size and platelet count, both at basal state and upon marrow regeneration. Similarly, in human samples, PIEZO1 is expressed during megakaryopoiesis. Its activation reduces Mk size, ploidy, maturation, and proplatelet extension. Resulting effects of PIEZO1 activation on Mks resemble the profile in Primary Myelofibrosis (PMF). Intriguingly, Mks derived from Jak2V617F PMF mice show significantly elevated PIEZO1 expression, compared to wild-type controls. Accordingly, Mks isolated from bone marrow aspirates of JAK2V617F PMF patients show increased PIEZO1 expression compared to Essential Thrombocythemia. Most importantly, PIEZO1 expression in bone marrow Mks is inversely correlated with patient platelet count. The ploidy, maturation, and proplatelet formation of Mks from JAK2V617F PMF patients are rescued upon PIEZO1 inhibition. Together, our data suggest that PIEZO1 places a brake on Mk maturation and platelet formation in physiology, and its upregulation in PMF Mks might contribute to aggravating some hallmarks of the disease.
© 2024 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of interest disclosure
All authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures

References
-
- Aguilar A, Pertuy F, Eckly A, et al. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
