The chemical logic of enzymatic lignin degradation
- PMID: 38165282
- PMCID: PMC10795516
- DOI: 10.1039/d3cc05298b
The chemical logic of enzymatic lignin degradation
Abstract
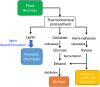
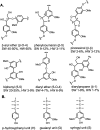
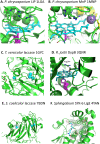
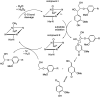
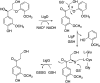
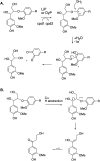
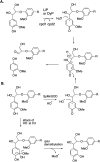
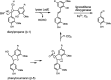
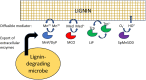
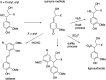
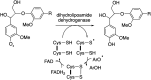
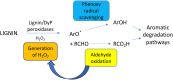
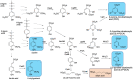
Lignin is an aromatic heteropolymer, found in plant cell walls as 20-30% of lignocellulose. It represents the most abundant source of renewable aromatic carbon in the biosphere, hence, if it could be depolymerised efficiently, then it would be a highly valuable source of renewable aromatic chemicals. However, lignin presents a number of difficulties for biocatalytic or chemocatalytic breakdown. Research over the last 10 years has led to the identification of new bacterial enzymes for lignin degradation, and the use of metabolic engineering to generate useful bioproducts from microbial lignin degradation. The aim of this article is to discuss the chemical mechanisms used by lignin-degrading enzymes and microbes to break down lignin, and to describe current methods for generating aromatic bioproducts from lignin using enzymes and engineered microbes.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
Sustainable production of aromatic chemicals from lignin using enzymes and engineered microbes.Chem Commun (Camb). 2024 Dec 3;60(97):14360-14375. doi: 10.1039/d4cc05064a. Chem Commun (Camb). 2024. PMID: 39569570 Free PMC article. Review.
-
The emerging role for bacteria in lignin degradation and bio-product formation.Curr Opin Biotechnol. 2011 Jun;22(3):394-400. doi: 10.1016/j.copbio.2010.10.009. Epub 2010 Nov 9. Curr Opin Biotechnol. 2011. PMID: 21071202
-
Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis.Biotechnol Biofuels. 2021 Apr 3;14(1):84. doi: 10.1186/s13068-021-01934-w. Biotechnol Biofuels. 2021. PMID: 33812391 Free PMC article. Review.
-
Enzymatic conversion of lignin into renewable chemicals.Curr Opin Chem Biol. 2015 Dec;29:10-7. doi: 10.1016/j.cbpa.2015.06.009. Epub 2015 Jun 26. Curr Opin Chem Biol. 2015. PMID: 26121945 Review.
-
The catabolism of lignin-derived p-methoxylated aromatic compounds by Rhodococcus jostii RHA1.Appl Environ Microbiol. 2024 Mar 20;90(3):e0215523. doi: 10.1128/aem.02155-23. Epub 2024 Feb 21. Appl Environ Microbiol. 2024. PMID: 38380926 Free PMC article.
Cited by
-
Unveiling the kinetic versatility of aryl-alcohol oxidases with different electron acceptors.Front Bioeng Biotechnol. 2024 Aug 5;12:1440598. doi: 10.3389/fbioe.2024.1440598. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39161354 Free PMC article.
-
Sustainable production of aromatic chemicals from lignin using enzymes and engineered microbes.Chem Commun (Camb). 2024 Dec 3;60(97):14360-14375. doi: 10.1039/d4cc05064a. Chem Commun (Camb). 2024. PMID: 39569570 Free PMC article. Review.
-
Lignin-Degrading Enzymes and the Potential of Pseudomonas putida as a Cell Factory for Lignin Degradation and Valorization.Microorganisms. 2025 Apr 18;13(4):935. doi: 10.3390/microorganisms13040935. Microorganisms. 2025. PMID: 40284771 Free PMC article. Review.
-
Fungal Coculture: Unlocking the Potential for Efficient Bioconversion of Lignocellulosic Biomass.J Fungi (Basel). 2025 Jun 17;11(6):458. doi: 10.3390/jof11060458. J Fungi (Basel). 2025. PMID: 40558970 Free PMC article. Review.
-
Aryl-alcohol oxidases: catalysis, diversity, structure-function and emerging biotechnological applications.Appl Microbiol Biotechnol. 2025 Jun 25;109(1):151. doi: 10.1007/s00253-025-13538-7. Appl Microbiol Biotechnol. 2025. PMID: 40560264 Free PMC article. Review.
References
-
- Fiorentino G. Ripa M. Ulgiati S. Biofuels, Bioprod. Biorefin. 2017;11:195–214. doi: 10.1002/bbb.1729. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

