MRI vs CT for Baseline Imaging Evaluation in Acute Large Artery Ischemic Stroke: A Subanalysis of the SWIFT-DIRECT Trial
- PMID: 38165324
- PMCID: PMC12337051
- DOI: 10.1212/WNL.0000000000207922
MRI vs CT for Baseline Imaging Evaluation in Acute Large Artery Ischemic Stroke: A Subanalysis of the SWIFT-DIRECT Trial
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Whether MRI or CT is preferable for the evaluation of patients with suspected stroke remains a matter of debate, given that the imaging modality acquired at baseline may be a relevant determinant of workflow delays and outcomes with it, in patients with stroke undergoing acute reperfusion therapies.
Methods: In this post hoc analysis of the SWIFT-DIRECT trial that investigated noninferiority of thrombectomy alone vs IV thrombolysis (IVT) + thrombectomy in patients with an acute ischemic anterior circulation large vessel occlusive stroke eligible to receive IVT within 4.5 hours after last seen well, we tested for a potential interaction between baseline imaging modality (MRI/MR-angiography [MRA] vs CT/CT-angiography [CTA]) and the effect of acute treatment (thrombectomy vs IVT + thrombectomy) on clinical and safety outcomes and procedural metrics (primary analysis). Moreover, we examined the association between baseline imaging modality and these outcomes using regression models adjusted for age, sex, baseline NIH Stroke Scale (NIHSS), occlusion location, and Alberta Stroke Program Early CT Score (ASPECTS) (secondary analysis). Endpoints included workflow times, the modified Rankin scale (mRS) score at 90 days, the rate of successful reperfusion, the odds for early neurologic deterioration within 24 hours, and the risk of symptomatic intracranial hemorrhage. The imaging modality acquired was chosen at the discretion of the treating physicians and commonly reflects center-specific standard procedures.
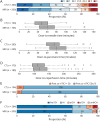
Results: Four hundred five of 408 patients enrolled in the SWIFT-DIRECT trial were included in this substudy. Two hundred (49.4%) patients underwent MRI/MRA, and 205 (50.6%) underwent CT/CTA. Patients with MRI/MRA had lower NIHSS scores (16 [interquartile range (IQR) 12-20] vs 18 [IQR 14-20], p = 0.012) and lower ASPECTS (8 [IQR 6-9] vs 8 [IQR 7-9], p = 0.021) compared with those with CT/CTA. In terms of the primary analysis, we found no evidence for an interaction between baseline imaging modality and the effect of IVT + thrombectomy vs thrombectomy alone. Regarding the secondary analysis, MRI/MRA acquisition was associated with workflow delays of approximately 20 minutes, higher odds of functional independence at 90 days (adjusted odds ratio [aOR] 1.65, 95% CI 1.07-2.56), and similar mortality rates (aOR 0.73, 95% CI 0.36-1.47) compared with CT/CTA.
Discussion: This post hoc analysis does not suggest treatment effect heterogeneity of IVT + thrombectomy vs thrombectomy alone in large artery stroke patients with different imaging modalities. There was no evidence that functional outcome at 90 days was less favorable following MRI/MRA at baseline compared with CT/CTA, despite significant workflow delays.
Trial registration information: ClinicalTrials.gov Identifier: NCT03192332.
Conflict of interest statement
J. Fladt reports no disclosures relevant to the manuscript. J. Kaesmacher reports funding from the Swiss National Science Society, Swiss Stroke Society, Bangerter-Rhyner foundation, and the CTU Bern. T. Meinel, L. Buetikofer, D. Strbian, O. Eker, J.F. Albucher, H. Desal, G. Marnat, C. Papagiannaki, S. Richard, M. Requena, B. Lapergue, P. Pagano, M. Ernst, M. Wiesmann, and M. Boulanger report no disclosures relevant to the manuscript. D.S. Liebeskind is a consultant to Cerenovus, Genentech, Medtronic, Stryker, and Rapid Medical. J. Gralla is the global PI of STAR (NCT01327989) and Swift Direct (NCT03192332) (Medtronic), Consultancy for Cerenovus-J&J Medtech Swiss National Foundation SNF grant for MRI in stroke; U. Fischer reports research support of the Swiss National Science Foundation and the Swiss Heart Foundation; PI of the ELAN trial and Co-PI of the DISTAL, TECNO, SWIFT DIRECT, and SWITCH trials; research grants from Medtronic (BEYOND SWIFT, SWIFT DIRECT) and from Stryker, Rapid medical, Penumbra and Phenox (DISTAL); consultancies for Medtronic, Stryker, and CSL Behring (fees paid to institution); participation in an advisory board for Alexion/Portola, Boehringer Ingelheim, Biogen, and Acthera (fees paid to institution); member of a clinical event committee (CEC) of the COATING study (Phenox) and member of the data and safety monitoring committee (DSMB) of the TITAN, LATE_MT, and IN EXTREMIS trials; vice-presidency of the Swiss Neurological Society. Go to
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
