Association Between Hippocampal Volumes and Cognition in Cerebral Amyloid Angiopathy
- PMID: 38165326
- PMCID: PMC10870737
- DOI: 10.1212/WNL.0000000000207854
Association Between Hippocampal Volumes and Cognition in Cerebral Amyloid Angiopathy
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Accumulating evidence suggests that gray matter atrophy, often considered a marker of Alzheimer disease (AD), can also result from cerebral small vessel disease (CSVD). Cerebral amyloid angiopathy (CAA) is a form of sporadic CSVD, diagnosed through neuroimaging criteria, that often co-occurs with AD pathology and leads to cognitive impairment. We sought to identify the role of hippocampal integrity in the development of cognitive impairment in a cohort of patients with possible and probable CAA.
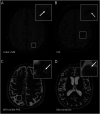
Methods: Patients were recruited from an ongoing CAA study at Massachusetts General Hospital. Composite scores defined performance in the cognitive domains of memory, language, executive function, and processing speed. Hippocampal subfields' volumes were measured from 3T MRI, using an automated method, and multivariate linear regression models were used to estimate their association with each cognitive domain and relationship to CAA-related neuroimaging markers.
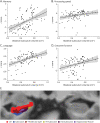
Results: One hundred twenty patients, 36 with possible (age mean [range]: 75.6 [65.6-88.9]), 67 with probable CAA (75.9 [59.0-94.0]), and 17 controls without cognitive impairment and CSVD (72.4 [62.5-82.7]; 76.4% female patients), were included in this study. We found a positive association between all investigated hippocampal subfields and memory and language, whereas specific subfields accounted for executive function (CA4 [Estimate = 5.43; 95% CI 1.26-9.61; p = 0.020], subiculum [Estimate = 2.85; 95% CI 0.67-5.02; p = 0.022]), and processing speed (subiculum [Estimate = 1.99; 95% CI 0.13-3.85; p = 0.036]). These findings were independent of other CAA-related markers, which did not have an influence on cognition in this cohort. Peak width of skeletonized mean diffusivity (PSMD), a measure of white matter integrity, was negatively associated with hippocampal subfields' volumes (CA3 [Estimate = -0.012; 95% CI -0.020 to -0.004; p = 0.034], CA4 [Estimate = -0.010; 95% CI -0.020 to -0.0007; p = 0.037], subiculum [Estimate = -0.019; 95% CI -0.042 to -0.0001; p = 0.003]).
Discussion: These results suggest that hippocampal integrity is an independent contributor to cognitive impairment in patients with CAA and that it might be related to loss of integrity in the white matter. Further studies exploring potential causes and directionality of the relationship between white matter and hippocampal integrity may be warranted.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
