Comparative Risk of Major Congenital Malformations With Antiseizure Medication Combinations vs Valproate Monotherapy in Pregnancy
- PMID: 38165339
- PMCID: PMC10870741
- DOI: 10.1212/WNL.0000000000207996
Comparative Risk of Major Congenital Malformations With Antiseizure Medication Combinations vs Valproate Monotherapy in Pregnancy
Abstract
Background and objectives: Valproate should be avoided in pregnancy, but it is the most effective drug for generalized epilepsies. Alternative treatment may require combinations of other drugs. Our objectives were to describe first trimester use of antiseizure medication (ASM) combinations that are relevant alternatives to valproate and determine whether specific combinations were associated with a lower risk of major congenital malformations (MCM) compared with valproate monotherapy.
Methods: We conducted a population-based cohort study using linked national registers from Denmark, Finland, Iceland, Norway, and Sweden and administrative health care data from the United States and New South Wales, Australia. We described first trimester use of ASM combinations among pregnant people with epilepsy from 2000 to 2020. We compared the risk of MCM after first trimester exposure to ASM combinations vs valproate monotherapy and low-dose valproate plus lamotrigine or levetiracetam vs high-dose valproate (≥1,000 mg/d). We used log-binomial regression with propensity score weights to calculate adjusted risk ratios (aRRs) and 95% CIs for each dataset. Results were pooled using fixed-effects meta-analysis.
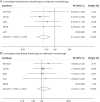
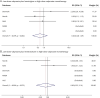
Results: Among 50,905 pregnancies in people with epilepsy identified from 7.8 million total pregnancies, 788 used lamotrigine and levetiracetam, 291 used lamotrigine and topiramate, 208 used levetiracetam and topiramate, 80 used lamotrigine and zonisamide, and 91 used levetiracetam and zonisamide. After excluding pregnancies with use of other ASMs, known teratogens, or a child diagnosed with MCM of infectious or genetic cause, we compared 587 exposed to lamotrigine-levetiracetam duotherapy and 186 exposed to lamotrigine-topiramate duotherapy with 1959 exposed to valproate monotherapy. Pooled aRRs were 0.41 (95% CI 0.24-0.69) and 1.26 (0.71-2.23), respectively. Duotherapy combinations containing low-dose valproate were infrequent, and comparisons with high-dose valproate monotherapy were inconclusive but suggested a lower risk for combination therapy. Other combinations were too rare for comparative safety analyses.
Discussion: Lamotrigine-levetiracetam duotherapy in first trimester was associated with a 60% lower risk of MCM than valproate monotherapy, while lamotrigine-topiramate was not associated with a reduced risk. Duotherapy with lamotrigine and levetiracetam may be favored to treat epilepsy in people with childbearing potential compared with valproate regarding MCM, but whether this combination is as effective as valproate remains to be determined.
Classification of evidence: This study provides Class II evidence that in people with epilepsy treated in the first trimester of pregnancy, the risk of major congenital malformations is lower with lamotrigine-levetiracetam duotherapy than with valproate alone, but similar with lamotrigine-topiramate.
Conflict of interest statement
J.M. Cohen reports no disclosures relevant to the manuscript; S. Alvestad reports speakers honoraria from Eisai; E.A. Suarez reports no disclosures relevant to the manuscript; A. Schaffer reports no disclosures relevant to the manuscript; R.M. Selmer reports no disclosures relevant to the manuscript; A. Havard reports no disclosures relevant to the manuscript; B.T. Bateman reports no disclosures relevant to the manuscript; C.E. Cesta reports participation in research projects funded by pharmaceutical companies, all regulator-mandated phase 4 studies, all with funds paid to the institution where she is employed (no personal fees) and with no relation to the work reported in this manuscript; H. Zoega reports no disclosures relevant to the manuscript; I. Odsbu reports participation in research projects funded by pharmaceutical companies, all regulator-mandated phase 4 studies, all with funds paid to the institution where she is employed (no personal fees) and with no relation to the work reported in this manuscript; K.F. Huybrechts reports research funding to her institution from Takeda and UCB, unrelated to this study; L.J. Kjerpeseth reports no disclosures relevant to the manuscript; L. Straub reports no disclosures relevant to the manuscript; M.K. Leinonen reports a grant from the Innovative Medicines Initiative (Building an ecosystem for better monitoring and communicating the safety of medicines use in pregnancy and breastfeeding: validated and regulatory endorsed workflows for fast, optimized evidence generation, IMI ConcePTION, grant agreement number 821520) while conducting the study; M.H. Bjørk reports funding to her institution from marketing authorization holders of valproate for a postauthorization safety study of valproate; M. Nørgaard reports no disclosures relevant to the manuscript; M. Gissler reports a grant from the Innovative Medicines Initiative (Building an ecosystem for better monitoring and communicating the safety of medicines use in pregnancy and breastfeeding: validated and regulatory endorsed workflows for fast, optimized evidence generation, IMI ConcePTION, grant agreement number 821520) while conducting the study; S.P. Ulrichsen reports no disclosures relevant to the manuscript; S. Hernandez-Diaz reports a research grant to her institution from Takeda and consulting fees from J&J and UCB for unrelated work; T. Tomson reports financial support to the EURAP International Antiepileptic Drugs and Pregnancy Registry from Accord, Angelini, Bial, Eisai, EcuPharma, GW Pharma, Glenmark, GSK, Sanofi, Teva, UCB, and Zentiva and speakers honoraria to his institution from Angelini, GSK, Eisai, and UCB; K. Furu reports no disclosures relevant to the manuscript. Go to
Figures
References
-
- Health Canada. Valproate anti-epileptic drugs may pose risks to children when taken by mothers during pregnancy [online]. Accessed April 12, 2019. healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2011/13625a-eng.php.
-
- U.S. Food & Drug Administration. FDA Drug Safety Communication: children born to mothers who took Valproate products while pregnant may have impaired cognitive development [6-30-2011] [online]. Accessed December 3, 2018. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communica...
-
- U.S. Food & Drug Administration. FDA Drug Safety Communication: valproate anti-seizure products contraindicated for migraine prevention in pregnant women due to decreased IQ scores in exposed children [05-06-2013] [online]. Accessed December 3, 2018. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communica...
-
- European Medicines Agency. PRAC recommends strengthening the restrictions on the use of valproate in women and girls [EMA/612389/2014] [online]. Accessed March 12, 2018. www.ema.europa.eu/en/documents/press-release/prac-recommends-strengtheni...
-
- European Medicines Agency. New measures to avoid valproate exposure in pregnancy endorsed [EMA/375438/2018] [online]. Accessed March 12, 2018. www.ema.europa.eu/en/documents/press-release/new-measures-avoid-valproat...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
