Association of Spinal Cord Atrophy and Brain Paramagnetic Rim Lesions With Progression Independent of Relapse Activity in People With MS
- PMID: 38165377
- PMCID: PMC10834139
- DOI: 10.1212/WNL.0000000000207768
Association of Spinal Cord Atrophy and Brain Paramagnetic Rim Lesions With Progression Independent of Relapse Activity in People With MS
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Progression independent of relapse activity (PIRA) is a crucial determinant of overall disability accumulation in multiple sclerosis (MS). Accelerated brain atrophy has been shown in patients experiencing PIRA. In this study, we assessed the relation between PIRA and neurodegenerative processes reflected by (1) longitudinal spinal cord atrophy and (2) brain paramagnetic rim lesions (PRLs). Besides, the same relationship was investigated in progressive MS (PMS). Last, we explored the value of cross-sectional brain and spinal cord volumetric measurements in predicting PIRA.
Methods: From an ongoing multicentric cohort study, we selected patients with MS with (1) availability of a susceptibility-based MRI scan and (2) regular clinical and conventional MRI follow-up in the 4 years before the susceptibility-based MRI. Comparisons in spinal cord atrophy rates (explored with linear mixed-effect models) and PRL count (explored with negative binomial regression models) were performed between: (1) relapsing-remitting (RRMS) and PMS phenotypes and (2) patients experiencing PIRA and patients without confirmed disability accumulation (CDA) during follow-up (both considering the entire cohort and the subgroup of patients with RRMS). Associations between baseline MRI volumetric measurements and time to PIRA were explored with multivariable Cox regression analyses.
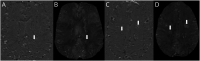
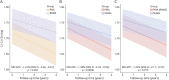
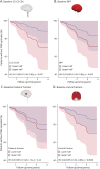
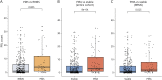
Results: In total, 445 patients with MS (64.9% female; mean [SD] age at baseline 45.0 [11.4] years; 11.2% with PMS) were enrolled. Compared with patients with RRMS, those with PMS had accelerated cervical cord atrophy (mean difference in annual percentage volume change [MD-APC] -1.41; p = 0.004) and higher PRL load (incidence rate ratio [IRR] 1.93; p = 0.005). Increased spinal cord atrophy (MD-APC -1.39; p = 0.0008) and PRL burden (IRR 1.95; p = 0.0008) were measured in patients with PIRA compared with patients without CDA; such differences were also confirmed when restricting the analysis to patients with RRMS. Baseline volumetric measurements of the cervical cord, whole brain, and cerebral cortex significantly predicted time to PIRA (all p ≤ 0.002).
Discussion: Our results show that PIRA is associated with both increased spinal cord atrophy and PRL burden, and this association is evident also in patients with RRMS. These findings further point to the need to develop targeted treatment strategies for PIRA to prevent irreversible neuroaxonal loss and optimize long-term outcomes of patients with MS.
Conflict of interest statement
A. Cagol, P. Benkert, L. Melie-Garcia, S.A. Schaedelin, S. Leber, C. Tsagkas, M. Barakovic, R. Galbusera, and P.-J. Lu have nothing to disclose. M. Weigel is partially funded by Biogen for the development of spinal cord MRI for patients with spinal muscular atrophy. E. Ruberte and E.-W. Radue have nothing to disclose. Ö. Yaldizli received grants from ECTRIMS/MAGNIMS, University of Basel, Pro Patient Stiftung University Hospital Basel, Free Academy Basel, Swiss Multiple Sclerosis Society and advisory board/lecture and consultancy fees from Roche, Sanofi Genzyme, Allmirall, Biogen and Novartis. J. Oechtering received research support by the Swiss MS Society and served on advisory boards for Roche and Merck. J. Lorscheider has received research support from Innosuisse-Swiss Innovation Agency, Biogen and Novartis, and speaking honoraria and/or fees for serving on advisory boards from Novartis, Roche and Teva. M. D'Souza has nothing to disclose in relationship to this work. B. Fisher-Barnicol has nothing to disclose. S. Müller received honoraria for travel, honoraria for lectures/consulting, and/or grants for studies from Almirall, Biogen, Celgene, Novartis, Teva, Merck Serono, Genzyme, Roche, and Bayer Schweiz. L. Achtnichts has nothing to disclose. J. Vehoff received honoraria for travel, honoraria for lectures/consulting and/or grants for studies from Allmiral, Biogen, Novartis, Teva, Merck-Serono, Genzyme, Roche and Bayer Schweiz AG, none related to this work. G. Disanto and O. Findling have nothing to disclose. A. Chan has served on advisory boards for, and received funding for travel or speaker honoraria from, Actelion-Janssen, Almirall, Bayer, Biogen, Celgene, Sanofi-Genzyme, Merck, Novartis, Roche, and Teva, all for hospital research funds; and research support from Biogen, Genzyme and UCB. A. Chan is associate editor of the
Figures
References
-
- Kappos L, Wolinsky JS, Giovannoni G, et al. . Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. doi:10.1001/jamaneurol.2020.1568 - DOI - PMC - PubMed
