Transmembrane Protein CMTM6 Alleviates Ocular Inflammatory Response and Improves Corneal Epithelial Barrier Function in Experimental Dry Eye
- PMID: 38165704
- PMCID: PMC10768713
- DOI: 10.1167/iovs.65.1.4
Transmembrane Protein CMTM6 Alleviates Ocular Inflammatory Response and Improves Corneal Epithelial Barrier Function in Experimental Dry Eye
Abstract
Purpose: To investigate the impact of transmembrane protein CMTM6 on the pathogenesis of dry eye disease (DED) and elucidate its potential mechanisms.
Methods: CMTM6 expression was confirmed by database analysis, real-time polymerase chain reaction (RT-PCR), western blot, and immunohistochemistry. Tear secretion was measured using the phenol red thread test. Immune cell infiltration was assessed through flow cytometry. Barrier function was evaluated by fluorescein sodium staining, immunofluorescence staining of zonula occludens 1 (ZO-1), and electric cell-substrate impedance sensing (ECIS) assessment. For silencing CMTM6 expression, siRNA and shRNA were employed, along with lentiviral vector-mediated overexpression of CMTM6. Proinflammatory cytokine levels were analyzed by RT-PCR and cytometric bead array (CBA) analysis.
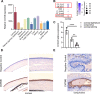
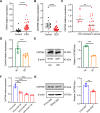
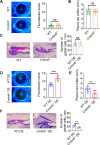
Results: CMTM6 showed high expression in healthy human and mouse corneal and conjunctival epithelium but was notably reduced in DED. Notably, this downregulation was correlated with disease severity. Cmtm6-/- dry eye (DE) mice displayed reduced tear secretion, severe corneal epithelial defects, decreased conjunctival goblet cell density, and upregulated inflammatory response. Additionally, Cmtm6-/- DE mice and CMTM6 knockdown human corneal epithelial cell-transformed (HCE-T) cells showed more severe barrier disruption and reduced expression of ZO-1. Knockdown of CMTM6 in HCE-T cells increased inflammatory responses induced by hyperosmotic stress, which was significantly mitigated by CMTM6 overexpression. Moreover, the level of phospho-p65 in hyperosmolarity-stimulated HCE-T cells increased after silencing CMTM6. Nuclear factor kappa B (NF-κB) p65 inhibition (JSH-23) reversed the excessive inflammatory responses caused by hyperosmolarity in CMTM6 knockdown HCE-T cells.
Conclusions: The reduction in CMTM6 expression on the ocular surface contributes to the pathogenesis of DED. The CMTM6-NF-κB p65 signaling pathway may serve as a promising therapeutic target for DED.
Conflict of interest statement
Disclosure:
Figures






Similar articles
-
Hyperosmotic stress-induced NLRP3 inflammasome activation via the mechanosensitive PIEZO1 channel in dry eye corneal epithelium.Ocul Surf. 2025 Apr;36:106-118. doi: 10.1016/j.jtos.2025.01.005. Epub 2025 Jan 18. Ocul Surf. 2025. PMID: 39832672
-
Protection of human corneal epithelial cells from TNF-α-induced disruption of barrier function by rebamipide.Invest Ophthalmol Vis Sci. 2013 Apr 17;54(4):2572-760. doi: 10.1167/iovs.12-11294. Invest Ophthalmol Vis Sci. 2013. PMID: 23482463
-
0.005% Preservative-Free Latanoprost Induces Dry Eye-Like Ocular Surface Damage via Promotion of Inflammation in Mice.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3375-3384. doi: 10.1167/iovs.18-24013. Invest Ophthalmol Vis Sci. 2018. PMID: 30025085
-
TRPV1 in Dry Eye Disease.Front Biosci (Landmark Ed). 2024 May 7;29(5):175. doi: 10.31083/j.fbl2905175. Front Biosci (Landmark Ed). 2024. PMID: 38812310 Review.
-
Molecular Mechanisms Responsible for Mesenchymal Stem Cell-Dependent Attenuation of Tear Hyperosmolarity and Immune Cell-Driven Inflammation in the Eyes of Patients with Dry Eye Disease.Diseases. 2024 Oct 26;12(11):269. doi: 10.3390/diseases12110269. Diseases. 2024. PMID: 39589943 Free PMC article. Review.
Cited by
-
Reticulated Retinoic Acid Synthesis is Implicated in the Pathogenesis of Dry Eye in Aqp5 Deficiency Mice.Invest Ophthalmol Vis Sci. 2024 Jul 1;65(8):25. doi: 10.1167/iovs.65.8.25. Invest Ophthalmol Vis Sci. 2024. PMID: 39017635 Free PMC article.
References
-
- Craig JP, Nichols KK, Akpek EK, et al. .. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017; 15(3): 276–283. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. .. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017; 15(3): 334–365. - PubMed
-
- Morthen MK, Magno MS, Utheim TP, Hammond CJ, Vehof J.. The work-related burden of dry eye. Ocul Surf. 2023; 28: 30–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

