Acrizanib as a Novel Therapeutic Agent for Fundus Neovascularization via Inhibitory Phosphorylation of VEGFR2
- PMID: 38165719
- PMCID: PMC10768700
- DOI: 10.1167/tvst.13.1.1
Acrizanib as a Novel Therapeutic Agent for Fundus Neovascularization via Inhibitory Phosphorylation of VEGFR2
Abstract
Purpose: The present study aimed to evaluate the effect of acrizanib, a small molecule inhibitor targeting vascular endothelial growth factor receptor 2 (VEGFR2), on physiological angiogenesis and pathological neovascularization in the eye and to explore the underlying molecular mechanisms.
Methods: We investigated the potential role of acrizanib in physiological angiogenesis using C57BL/6J newborn mice, and pathological angiogenesis using the mouse oxygen-induced retinopathy (OIR) and laser-induced choroidal neovascularization (CNV) models. Moreover, vascular endothelial growth factor (VEGF)-treated human umbilical vein endothelial cells (HUVECs) were used as an in vitro model for studying the molecular mechanism underlying acrizanib's antiangiogenic effects.
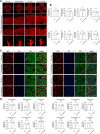
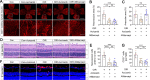
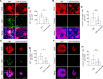
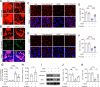
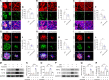
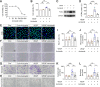
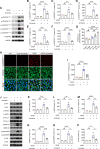
Results: The intravitreal injection of acrizanib did not show a considerable impact on physiological angiogenesis and retinal thickness, indicating a potentially favorable safety profile. In the mouse models of OIR and CNV, acrizanib showed promising results in reducing pathological neovascularization, inflammation, and vascular leakage, indicating its potential efficacy against pathological angiogenesis. Consistent with in vivo results, acrizanib blunted angiogenic events in VEGF-treated HUVECs such as proliferation, migration, and tube formation. Furthermore, acrizanib inhibited the multisite phosphorylation of VEGFR2 to varying degrees and the activation of its downstream signal pathways in VEGF-treated HUVECs.
Conclusions: This study suggested the potential efficacy and safety of acrizanib in suppressing fundus neovascularization. Acrizanib functioned through inhibiting multiple phosphorylation sites of VEGFR2 in endothelial cells to different degrees.
Translational relevance: These results indicated that acrizanib might hold promise as a potential candidate for the treatment of ocular vascular diseases.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Advances in the molecular signaling mechanisms of VEGF/VEGFR2 in fundus neovascularization disease (Review).Exp Ther Med. 2025 May 20;30(1):143. doi: 10.3892/etm.2025.12893. eCollection 2025 Jul. Exp Ther Med. 2025. PMID: 40496761 Free PMC article. Review.
-
Apatinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor 2, Suppresses Pathologic Ocular Neovascularization in Mice.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3592-3599. doi: 10.1167/iovs.17-21416. Invest Ophthalmol Vis Sci. 2017. PMID: 28715845
-
Knockout of αA-crystallin inhibits ocular neovascularization.Invest Ophthalmol Vis Sci. 2015 Jan 8;56(2):816-26. doi: 10.1167/iovs.14-14734. Invest Ophthalmol Vis Sci. 2015. PMID: 25574047
-
Antiangiogenic effect of dasatinib in murine models of oxygen-induced retinopathy and laser-induced choroidal neovascularization.Mol Vis. 2017 Nov 24;23:823-831. eCollection 2017. Mol Vis. 2017. PMID: 29225458 Free PMC article.
-
Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide.Biomedicines. 2024 Dec 15;12(12):2851. doi: 10.3390/biomedicines12122851. Biomedicines. 2024. PMID: 39767758 Free PMC article. Review.
Cited by
-
Advances in the molecular signaling mechanisms of VEGF/VEGFR2 in fundus neovascularization disease (Review).Exp Ther Med. 2025 May 20;30(1):143. doi: 10.3892/etm.2025.12893. eCollection 2025 Jul. Exp Ther Med. 2025. PMID: 40496761 Free PMC article. Review.
-
Inhibition of Endothelial HIF-1α by IDF-11774 Attenuates Retinal Neovascularization and Vascular Leakage.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):47. doi: 10.1167/iovs.66.9.47. Invest Ophthalmol Vis Sci. 2025. PMID: 40668060 Free PMC article.
-
The Diagnosis and Treatment of Branch Retinal Vein Occlusions: An Update.Biomedicines. 2025 Jan 5;13(1):105. doi: 10.3390/biomedicines13010105. Biomedicines. 2025. PMID: 39857689 Free PMC article. Review.
References
-
- Cheung N, Mitchell P, Wong TY.. Diabetic retinopathy. Lancet. 2010; 376: 124–136. - PubMed
-
- Mitchell P, Liew G, Gopinath B, et al. .. Age-related macular degeneration. Lancet. 2018; 392: 1147–1159. - PubMed
-
- Selvam S, Kumar T, Fruttiger M.. Retinal vasculature development in health and disease. Prog Retin Eye Res. 2018; 63: 1–19. - PubMed
-
- Grossniklaus HE, Green WR.. Choroidal neovascularization. Am J Ophthalmol. 2004; 137: 496–503. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

