Clinical factors associated with the therapeutic efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma: A multicenter prospective observational study
- PMID: 38165900
- PMCID: PMC10760712
- DOI: 10.1371/journal.pone.0294590
Clinical factors associated with the therapeutic efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma: A multicenter prospective observational study
Abstract
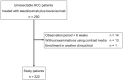
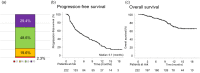
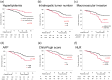
The treatment efficiency and predictors of atezolizumab plus bevacizumab therapy for unresectable hepatocellular carcinoma in real-world practice have not been established. This study aimed to assess the efficacy and safety of atezolizumab plus bevacizumab and to investigate predictors of progression-free survival and overall survival. Patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab therapy in 19 hospitals were enrolled before treatment and observed prospectively. The outcomes of 222 patients in this cohort were analyzed. The objective response rate and disease control rate were 22.0% and 70.6%, respectively, whereas the median progression-free survival was 5.7 months. Independent risk factors for shortened progression-free survival were younger age (<75 years; 3.9 months vs. 8.6 months), higher number of intrahepatic tumors (≥5; 4.0 months vs. 7.9 months), macrovascular invasion (2.3 months vs. 6.7 months), and higher neutrophil-to-lymphocyte ratio (≥3.03; 3.0 months vs. 7.8 months). The median overall survival was not reached; however, independent risk factors for shortened overall survival were absence of hyperlipidemia, higher number of intrahepatic tumors (≥5), macrovascular invasion, higher α-fetoprotein level (≥400 ng/mL), worse Child-Pugh score (≥6), and higher neutrophil-to-lymphocyte ratio (≥3.03). Severe adverse events (grade ≥3) were observed in 96 patients (36.0%), with proteinuria being the most frequent. In conclusion, patients with older age, lower number of intrahepatic tumors, absent macrovascular invasion, and lower neutrophil-to-lymphocyte ratio are expected to have better progression-free survival with atezolizumab plus bevacizumab therapy for unresectable hepatocellular carcinoma.
Copyright: © 2024 Kai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Tetsuo Takehara has received lecture fees and research grants from Chugai Pharmaceutical Co., Ltd, Eisai Co., Ltd. Takahiro Kodama has received lecture fees from Chugai Pharmaceutical Co., Ltd and AstraZeneca K.K. All other authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

