Oxazolone mediated peptide chain extension and homochirality in aqueous microdroplets
- PMID: 38165938
- PMCID: PMC10786291
- DOI: 10.1073/pnas.2309360120
Oxazolone mediated peptide chain extension and homochirality in aqueous microdroplets
Abstract
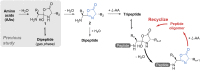
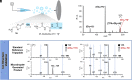
Peptide formation from amino acids is thermodynamically unfavorable but a recent study provided evidence that the reaction occurs at the air/solution interfaces of aqueous microdroplets. Here, we show that i) the suggested amino acid complex in microdroplets undergoes dehydration to form oxazolone; ii) addition of water to oxazolone forms the dipeptide; and iii) reaction of oxazolone with other amino acids forms tripeptides. Furthermore, the chirality of the reacting amino acids is preserved in the oxazolone product, and strong chiral selectivity is observed when converting the oxazolone to tripeptide. This last fact ensures that optically impure amino acids will undergo chain extension to generate pure homochiral peptides. Peptide formation in bulk by wet-dry cycling shares a common pathway with the microdroplet reaction, both involving the oxazolone intermediate.
Keywords: microdroplet chemistry; origin of homochirality; origin of life; peptide formation; prebiotic chemistry.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
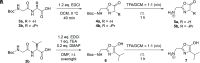



Similar articles
-
Serine Octamer Clusters Direct the Chirality of Peptides Formed in Water Microdroplets.J Phys Chem A. 2025 May 22;129(20):4535-4542. doi: 10.1021/acs.jpca.5c00473. Epub 2025 May 8. J Phys Chem A. 2025. PMID: 40340402 Free PMC article.
-
Aqueous microdroplets enable abiotic synthesis and chain extension of unique peptide isomers from free amino acids.Proc Natl Acad Sci U S A. 2022 Oct 18;119(42):e2212642119. doi: 10.1073/pnas.2212642119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191178 Free PMC article.
-
Water Microdroplets Allow Spontaneously Abiotic Production of Peptides.J Phys Chem Lett. 2021 Jun 24;12(24):5774-5780. doi: 10.1021/acs.jpclett.1c01083. Epub 2021 Jun 16. J Phys Chem Lett. 2021. PMID: 34134488
-
Spontaneous Oxidation in Aqueous Microdroplets: Water Radical Cation as Primary Oxidizing Agent.Angew Chem Int Ed Engl. 2024 Apr 22;63(17):e202400118. doi: 10.1002/anie.202400118. Epub 2024 Feb 19. Angew Chem Int Ed Engl. 2024. PMID: 38302696 Review.
-
Biological Homochirality and the Search for Extraterrestrial Biosignatures.Orig Life Evol Biosph. 2022 Sep;52(1-3):93-104. doi: 10.1007/s11084-022-09623-w. Epub 2022 Aug 15. Orig Life Evol Biosph. 2022. PMID: 35969306 Review.
Cited by
-
Accelerated peptide bond formation at air-water interfaces.Proc Natl Acad Sci U S A. 2025 Mar 25;122(12):e2501323122. doi: 10.1073/pnas.2501323122. Epub 2025 Mar 21. Proc Natl Acad Sci U S A. 2025. PMID: 40117307
-
Interfacial electromigration for accelerated reactions.Anal Chim Acta. 2025 May 1;1349:343854. doi: 10.1016/j.aca.2025.343854. Epub 2025 Feb 22. Anal Chim Acta. 2025. PMID: 40074462 Free PMC article.
-
Accelerated click reactions using boronic acids for heterocyclic synthesis in microdroplets.Chem Sci. 2025 Apr 10;16(20):8800-8806. doi: 10.1039/d5sc00851d. eCollection 2025 May 21. Chem Sci. 2025. PMID: 40276635 Free PMC article.
-
Serine Octamer Clusters Direct the Chirality of Peptides Formed in Water Microdroplets.J Phys Chem A. 2025 May 22;129(20):4535-4542. doi: 10.1021/acs.jpca.5c00473. Epub 2025 May 8. J Phys Chem A. 2025. PMID: 40340402 Free PMC article.
-
Abiotic formation of hexoses and disaccharides in aqueous microdroplets.Chem Sci. 2025 Mar 18;16(16):7057-7065. doi: 10.1039/d4sc08402k. eCollection 2025 Apr 16. Chem Sci. 2025. PMID: 40144502 Free PMC article.
References
-
- Cronin J. R. R., Pizzarello S., Amino acids in meteorites. Adv. Sp. Res. 3, 5–18 (1983). - PubMed
-
- Brack A., From interstellar amino acids to prebiotic catalytic peptides: A review. Chem. Biodivers. 4, 665–679 (2007). - PubMed
-
- Pizzarello S., Cooper G. W., Flynn G. J., “The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles” in Meteorites and the Early Solar System II, D. S. Lauretta, H. Y. McSween, Eds. (University of Arizona Press, 2021), pp. 625–652.
-
- Deal A. M., Rapf R. J., Vaida V., Water-air interfaces as environments to address the water paradox in prebiotic chemistry: A physical chemistry perspective. J. Phys. Chem. A 125, 4929–4942 (2021). - PubMed
-
- Sutherland J. D., Opinion: Studies on the origin of life-the end of the beginning. Nat. Rev. Chem. 1, 1–8 (2017).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

