Role of Influenza A virus protein NS1 in regulating host nuclear body ND10 complex formation and its involvement in establishment of viral pathogenesis
- PMID: 38166085
- PMCID: PMC10760828
- DOI: 10.1371/journal.pone.0295522
Role of Influenza A virus protein NS1 in regulating host nuclear body ND10 complex formation and its involvement in establishment of viral pathogenesis
Abstract
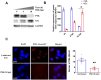
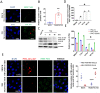
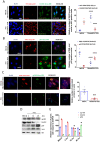
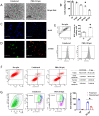
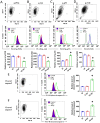
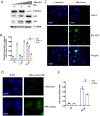
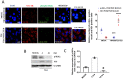
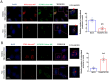
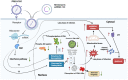
Influenza viral infection is a seasonal infection which causes widespread acute respiratory issues among humans globally. This virus changes its surface receptor composition to escape the recognition process by the host's immune cells. Therefore, the present study focussed to identify some other important viral proteins which have a significant role in establishment of infection and having apparent conserved structural composition. This could facilitate the permanent vaccine development process or help in designing a drug against IAV (influenza A virus) infection which will eliminate the seasonal flu shot vaccination process. The NS1 (Non-structural protein 1) protein of IAV maintains a conserved structural motif. Earlier studies have shown its significant role in infection establishment. However, the mechanism by which viruses escape the host's ND10 antiviral action remains elusive. The present study clearly showed that IAV infection and NS1 transfection in A549 cells degraded the main component of the ND10 anti-viral complex, PML and therefore, inhibited the formation of Daxx-sp100-p53-PML complex (ND10) at the mid phase of infection/transfection. PML degradation activated the stress axis which increased cellular ROS (reactive oxygen species) levels as well as mitochondrial dysfunction. Additionally, IAV/NS1 increased cellular stress and p53 accumulation at the late phase of infection. These collectively activated apoptotic pathway in the host cells. Along with the inactivation of several interferon proteins, IAV was found to decrease p-IKKε. A549 cells transfected with pcDNA3.1-NS1 showed a similar effect in the interferon axis and IKKε. Moreover, NS1 induced the disintegration of the host's ND10 complex through the changes in the SUMOylation pattern of the PML nuclear body. These findings suggest the possible mechanism of how NS1 helps IAV to establish infection in the host cells. However, it demands further detailed study before targeting NS1 to develop permanent vaccines or novel drugs against IAV in future.
Copyright: © 2024 Das et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4.J Virol. 2019 Mar 21;93(7):e02168-18. doi: 10.1128/JVI.02168-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651364 Free PMC article.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
Interplay of PA-X and NS1 Proteins in Replication and Pathogenesis of a Temperature-Sensitive 2009 Pandemic H1N1 Influenza A Virus.J Virol. 2017 Aug 10;91(17):e00720-17. doi: 10.1128/JVI.00720-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637750 Free PMC article.
-
Fluorescence-Activated Cell Sorting-Based Analysis Reveals an Asymmetric Induction of Interferon-Stimulated Genes in Response to Seasonal Influenza A Virus.J Virol. 2015 Jul;89(14):6982-93. doi: 10.1128/JVI.00857-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903337 Free PMC article.
-
Research progress on the nonstructural protein 1 (NS1) of influenza a virus.Virulence. 2024 Dec;15(1):2359470. doi: 10.1080/21505594.2024.2359470. Epub 2024 Jun 25. Virulence. 2024. PMID: 38918890 Free PMC article. Review.
Cited by
-
Bacteriocin-Mediated Silver Nanoconjugate: Synthesis, Characterization, and Application as an Antibiofilm Agent Against Two Common Pathogenic Bacteria.Probiotics Antimicrob Proteins. 2025 Jul 14. doi: 10.1007/s12602-025-10656-6. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40658290
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
-
Triggering Degradation of Host Cellular Proteins for Robust Propagation of Influenza Viruses.Int J Mol Sci. 2024 Apr 25;25(9):4677. doi: 10.3390/ijms25094677. Int J Mol Sci. 2024. PMID: 38731896 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention 2010. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010; 59:1057–1062. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

