Neuroprotective effect of the RNS60 in a mouse model of transient focal cerebral ischemia
- PMID: 38166102
- PMCID: PMC10760892
- DOI: 10.1371/journal.pone.0295504
Neuroprotective effect of the RNS60 in a mouse model of transient focal cerebral ischemia
Abstract
Background: Stroke is a major cause of death, disability, and public health problems. Its intervention is limited to early treatment with thrombolytics and/or endovascular clot removal with mechanical thrombectomy without any available subacute or chronic neuroprotective treatments. RNS60 has reduced neuroinflammation and increased neuronal survival in several animal models of neurodegeneration and trauma. The aim here was to evaluate whether RNS60 protects the brain and cognitive function in a mouse stroke model.
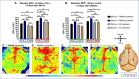
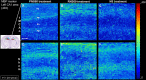
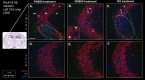
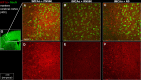
Methods: Male C57BL/6J mice were subjected to sham or ischemic stroke surgery using 60-minute transient middle cerebral artery occlusion (tMCAo). In each group, mice received blinded daily administrations of RNS60 or control fluids (PNS60 or normal saline [NS]), beginning 2 hours after surgery over 13 days. Multiple neurobehavioral tests were conducted (Neurological Severity Score [mNSS], Novel Object Recognition [NOR], Active Place Avoidance [APA], and the Conflict Variant of APA [APAc]). On day 14, cortical microvascular perfusion (MVP) was measured, then brains were removed and infarct volume, immunofluorescence of amyloid beta (Aβ), neuronal density, microglial activation, and white matter damage/myelination were measured. SPSS was used for analysis (e.g., ANOVA for parametric data; Kruskal Wallis for non-parametric data; with post-hoc analysis).
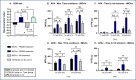
Results: Thirteen days of treatment with RNS60 reduced brain infarction, amyloid pathology, neuronal death, microglial activation, white matter damage, and increased MVP. RNS60 reduced brain pathology and resulted in behavioral improvements in stroke compared to sham surgery mice (increased memory-learning in NOR and APA, improved cognitive flexibility in APAc).
Conclusion: RNS60-treated mice exhibit significant protection of brain tissue and improved neurobehavioral functioning after tMCAo-stroke. Additional work is required to determine mechanisms, time-window of dosing, and multiple dosing volumes durations to support clinical stroke research.
Copyright: © 2024 Baena-Caldas et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist. Drs. SG and AK are full-time employees of the Revalesio Corporation but have not conflict of interest or any disclosures. They are authors because they participated in the experimental design, visualization, and manuscript preparation but did not participate in any data collection or analysis.
Figures


References
-
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

