Price negotiation and pricing of anticancer drugs in China: An observational study
- PMID: 38166148
- PMCID: PMC10793910
- DOI: 10.1371/journal.pmed.1004332
Price negotiation and pricing of anticancer drugs in China: An observational study
Abstract
Background: While China has implemented reimbursement-linked drug price negotiation annually since 2017, emphasizing value-based pricing to achieve a value-based strategic purchase of medical insurance, whether drug prices became better aligned with clinical value after price negotiation has not been sufficiently established. This study aimed to assess the changes in prices and their relationship with the clinical value of anticancer drugs after the implementation of price negotiations in China.
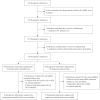
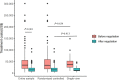
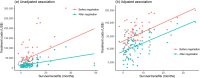
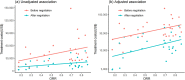
Methods and findings: In this observational study, anticancer drug indications that were negotiated successfully between 2017 and 2022 were identified through National Reimbursement Drug Lists (NRDL) of China. We excluded extensions of indications for drugs already listed in the NRDL, indications for pediatric use, and indications lacking corresponding clinical trials. We identified pivotal clinical trials for included indications by consulting review reports or drug labels issued by the Center for Drug Evaluation, National Medical Products Administration. We calculated treatment costs as outcome measures based on publicly available prices and collected data on clinical value including safety, survival, quality of life, and overall response rate (ORR) from publications of pivotal clinical trials. The associations between drug costs and clinical value, both before and after negotiation, were analyzed using regression analyses. We also examined whether price negotiation has led to a reduction in the variation of treatment costs for a given value. We included 103 anticancer drug indications, primarily for the treatment of blood cancer, lung cancer, and breast cancer, with 76 supported by randomized controlled trials and 27 supported by single-arm clinical trials. The median treatment costs over the entire sample have been reduced from US$34,460.72 (interquartile range (IQR): 19,990.49 to 55,441.66) to US$13,688.79 (IQR: 7,746.97 to 21,750.97) after price negotiation (P < 0.001). Before price negotiation, each additional month of survival gained was associated with an increase in treatment costs of 3.4% (95% confidence interval (CI) [2.1, 4.8], P < 0.001) for indications supported by randomized controlled trials, and a 10% increase in ORR was associated with a 6.0% (95% CI [1.6, 10.3], P = 0.009) increase in treatment costs for indications supported by single-arm clinical trials. After price negotiation, the associations between costs and clinical value may not have changed significantly, but the variation of drug costs for a given value was reduced. Study limitations include the lack of transparency in official data, missing data on clinical value, and a limited sample size.
Conclusions: In this study, we found that the implementation of price negotiation in China has led to drug pricing better aligned with clinical value for anticancer drugs even after substantial price reductions. The achievements made in China could shed light on the price regulation in other countries, particularly those with limited resources and increasing drug expenditures.
Copyright: © 2024 Zhou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA. Oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987 . - DOI - PMC - PubMed
-
- IQVIA institute. Global Oncology Trends 2023: Outlook to 2027. 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

