Elimination of Chlamydia muridarum from the female reproductive tract is IL-12p40 dependent, but independent of Th1 and Th2 cells
- PMID: 38166152
- PMCID: PMC10786385
- DOI: 10.1371/journal.ppat.1011914
Elimination of Chlamydia muridarum from the female reproductive tract is IL-12p40 dependent, but independent of Th1 and Th2 cells
Abstract
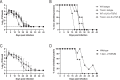
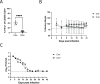
Chlamydia vaccine approaches aspire to induce Th1 cells for optimal protection, despite the fact that there is no direct evidence demonstrating Th1-mediated Chlamydia clearance from the female reproductive tract (FRT). We recently reported that T-bet-deficient mice can resolve primary Chlamydia infection normally, undermining the potentially protective role of Th1 cells in Chlamydia immunity. Here, we show that T-bet-deficient mice develop robust Th17 responses and that mice deficient in Th17 cells exhibit delayed bacterial clearance, demonstrating that Chlamydia-specific Th17 cells represent an underappreciated protective population. Additionally, Th2-deficient mice competently clear cervicovaginal infection. Furthermore, we show that sensing of IFN-γ by non-hematopoietic cells is essential for Chlamydia immunity, yet bacterial clearance in the FRT does not require IFN-γ secretion by CD4 T cells. Despite the fact that Th1 cells are not necessary for Chlamydia clearance, protective immunity to Chlamydia is still dependent on MHC class-II-restricted CD4 T cells and IL-12p40. Together, these data point to IL-12p40-dependent CD4 effector maturation as essential for Chlamydia immunity, and Th17 cells to a lesser extent, yet neither Th1 nor Th2 cell development is critical. Future Chlamydia vaccination efforts will be more effective if they focus on induction of this protective CD4 T cell population.
Copyright: © 2024 Rixon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021. Atlanta: U.S. Department of Health and Human Services; 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

