Ultrasound Stimulation of Piezoelectric Nanocomposite Hydrogels Boosts Chondrogenic Differentiation in Vitro, in Both a Normal and Inflammatory Milieu
- PMID: 38166155
- PMCID: PMC10811754
- DOI: 10.1021/acsnano.3c08738
Ultrasound Stimulation of Piezoelectric Nanocomposite Hydrogels Boosts Chondrogenic Differentiation in Vitro, in Both a Normal and Inflammatory Milieu
Abstract
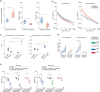
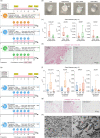
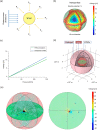
The use of piezoelectric nanomaterials combined with ultrasound stimulation is emerging as a promising approach for wirelessly triggering the regeneration of different tissue types. However, it has never been explored for boosting chondrogenesis. Furthermore, the ultrasound stimulation parameters used are often not adequately controlled. In this study, we show that adipose-tissue-derived mesenchymal stromal cells embedded in a nanocomposite hydrogel containing piezoelectric barium titanate nanoparticles and graphene oxide nanoflakes and stimulated with ultrasound waves with precisely controlled parameters (1 MHz and 250 mW/cm2, for 5 min once every 2 days for 10 days) dramatically boost chondrogenic cell commitment in vitro. Moreover, fibrotic and catabolic factors are strongly down-modulated: proteomic analyses reveal that such stimulation influences biological processes involved in cytoskeleton and extracellular matrix organization, collagen fibril organization, and metabolic processes. The optimal stimulation regimen also has a considerable anti-inflammatory effect and keeps its ability to boost chondrogenesis in vitro, even in an inflammatory milieu. An analytical model to predict the voltage generated by piezoelectric nanoparticles invested by ultrasound waves is proposed, together with a computational tool that takes into consideration nanoparticle clustering within the cell vacuoles and predicts the electric field streamline distribution in the cell cytoplasm. The proposed nanocomposite hydrogel shows good injectability and adhesion to the cartilage tissue ex vivo, as well as excellent biocompatibility in vivo, according to ISO 10993. Future perspectives will involve preclinical testing of this paradigm for cartilage regeneration.
Keywords: chondrogenesis; hydrogel; inflammation; mesenchymal stromal cell; nanomaterial; piezoelectric; ultrasound.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Dolai J.; Biswas A.; Jana N. R. Piezoelectric Nanoparticles for Ultrasound-Based Wireless Therapies. ACS Appl. Nano Mater. 2022, 5, 14038–14050. 10.1021/acsanm.2c03421. - DOI
-
- Ji J.; Yang C.; Shan Y.; Sun M.; Cui X.; Xu L.; Li Z.; et al. Research Trends of Piezoelectric Nanomaterials in Biomedical Engineering. Adv. NanoBiomed Res. 2023, 3, 2200088. 10.1002/anbr.202200088. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

