Cardiac MRI and Clinical Outcomes in TMEM43 Arrhythmogenic Cardiomyopathy
- PMID: 38166344
- PMCID: PMC11163247
- DOI: 10.1148/ryct.230155
Cardiac MRI and Clinical Outcomes in TMEM43 Arrhythmogenic Cardiomyopathy
Abstract
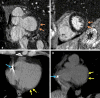
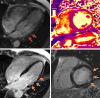
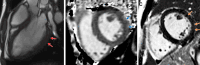
Arrhythmogenic cardiomyopathy is an inherited cardiomyopathy that can involve both ventricles. Several genes have been identified as pathogenic in arrhythmogenic cardiomyopathy, including TMEM43. However, there are limited data on cardiac MRI findings in patients with TMEM43 variants to date. In this case series, cardiac MRI findings and clinical outcomes are described in 14 patients with TMEM43 variants, including eight (57%) with the pathogenic p.Ser358Leu variant (six female patients; mean age, 33 years ± 15 [SD]) and six (43%) with a TMEM43 variant of unknown significance (three female patients; mean age, 38 years ± 11). MRI findings demonstrated left ventricular systolic dysfunction in eight (57%) patients and right ventricular dysfunction in four (29%) patients. Among the nine patients with late gadolinium enhancement imaging, left ventricular late gadolinium enhancement was present in seven (78%; all subepicardial) patients. In summary, TMEM43 variants are associated with high prevalence of subepicardial late gadolinium enhancement and left ventricular dysfunction. Keywords: Arrhythmogenic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy, TMEM43, Cardiac MRI, Genetic Variants Supplemental material is available for this article.
Keywords: Arrhythmogenic Cardiomyopathy; Arrhythmogenic Right Ventricular Cardiomyopathy; Cardiac MRI; Genetic Variants; TMEM43.
© RSNA, 2023.
Conflict of interest statement
Figures


Similar articles
-
Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy.Circulation. 2020 Jun 9;141(23):1872-1884. doi: 10.1161/CIRCULATIONAHA.119.044934. Epub 2020 May 6. Circulation. 2020. PMID: 32372669 Free PMC article.
-
[Arrhythmogenic cardiomyopathy. Patterns of ventricular involvement using cardiac magnetic resonance].Rev Esp Cardiol. 2011 Dec;64(12):1114-22. doi: 10.1016/j.recesp.2011.07.014. Epub 2011 Oct 24. Rev Esp Cardiol. 2011. PMID: 22030343 Spanish.
-
DSP p.(Thr2104Glnfs*12) variant presents variably with early onset severe arrhythmias and left ventricular cardiomyopathy.BMC Med Genet. 2020 Jan 31;21(1):19. doi: 10.1186/s12881-020-0955-z. BMC Med Genet. 2020. PMID: 32005173 Free PMC article.
-
Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy.J Am Heart Assoc. 2021 Sep 21;10(18):e021987. doi: 10.1161/JAHA.121.021987. Epub 2021 Sep 17. J Am Heart Assoc. 2021. PMID: 34533054 Free PMC article. Review.
-
Genetic Risk Stratification in Arrhythmogenic Left Ventricular Cardiomyopathy.Card Electrophysiol Clin. 2023 Sep;15(3):391-399. doi: 10.1016/j.ccep.2023.04.005. Epub 2023 Jun 20. Card Electrophysiol Clin. 2023. PMID: 37558308 Review.
Cited by
-
The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review.J Clin Med. 2025 Aug 8;14(16):5611. doi: 10.3390/jcm14165611. J Clin Med. 2025. PMID: 40869437 Free PMC article. Review.
-
The cGAS/STING Pathway: Friend or Foe in Regulating Cardiomyopathy.Cells. 2025 May 25;14(11):778. doi: 10.3390/cells14110778. Cells. 2025. PMID: 40497954 Free PMC article. Review.
-
Transmembrane Protein 43: Molecular and Pathogenetic Implications in Arrhythmogenic Cardiomyopathy and Various Other Diseases.Int J Mol Sci. 2025 Jul 17;26(14):6856. doi: 10.3390/ijms26146856. Int J Mol Sci. 2025. PMID: 40725103 Free PMC article. Review.
-
Cardiac Magnetic Resonance Guidance for the Pathogenetic Definition of Cardiomyopathies.Curr Cardiol Rep. 2025 Apr 16;27(1):85. doi: 10.1007/s11886-025-02233-8. Curr Cardiol Rep. 2025. PMID: 40238040 Free PMC article. Review.
References
-
- Corrado D , Basso C , Judge DP . Arrhythmogenic Cardiomyopathy . Circ Res 2017. ; 121 ( 7 ): 784 – 802 . - PubMed
-
- Marcus FI , Fontaine GH , Guiraudon G , et al. . Right ventricular dysplasia: a report of 24 adult cases . Circulation 1982. ; 65 ( 2 ): 384 – 398 . - PubMed
-
- Corrado D , Perazzolo Marra M , Zorzi A , et al. . Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria . Int J Cardiol 2020. ; 319 : 106 – 114 . - PubMed
-
- Fressart V , Duthoit G , Donal E , et al. . Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice . Europace 2010. ; 12 ( 6 ): 861 – 868 . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

