Modeling immunity in microphysiological systems
- PMID: 38166397
- PMCID: PMC10800123
- DOI: 10.1177/15353702231215897
Modeling immunity in microphysiological systems
Abstract
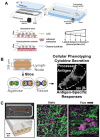
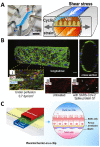
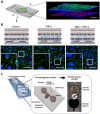
There is a need for better predictive models of the human immune system to evaluate safety and efficacy of immunomodulatory drugs and biologics for successful product development and regulatory approvals. Current in vitro models, which are often tested in two-dimensional (2D) tissue culture polystyrene, and preclinical animal models fail to fully recapitulate the function and physiology of the human immune system. Microphysiological systems (MPSs) that can model key microenvironment cues of the human immune system, as well as of specific organs and tissues, may be able to recapitulate specific features of the in vivo inflammatory response. This minireview provides an overview of MPS for modeling lymphatic tissues, immunity at tissue interfaces, inflammatory diseases, and the inflammatory tumor microenvironment in vitro and ex vivo. Broadly, these systems have utility in modeling how certain immunotherapies function in vivo, how dysfunctional immune responses can propagate diseases, and how our immune system can combat pathogens.
Keywords: Microphysiological systems; immunity; inflammatory diseases; lymphatics; tissue interfaces; tumor microenvironment.
Copyright © 2024 by the Society for Experimental Biology and Medicine
Conflict of interest statement
Declaration Of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Descotes JJ. Methods of evaluating immunotoxicity. Expert Opin Drug Metab Toxicol 2006;2:249–59 - PubMed
-
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010;10:735–44 - PubMed
-
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg 2004;187:11S–6 - PubMed
-
- Zschaler JJ, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 2014;34:433–54 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

