A novel pathogenic variant in the carnitine transporter gene, SLC22A5, in association with metabolic carnitine deficiency and cardiomyopathy features
- PMID: 38166572
- PMCID: PMC10763261
- DOI: 10.1186/s12872-023-03676-z
A novel pathogenic variant in the carnitine transporter gene, SLC22A5, in association with metabolic carnitine deficiency and cardiomyopathy features
Abstract
Background: Primary carnitine deficiency (PCD) denotes low carnitine levels with an autosomal recessive pattern of inheritance. Cardiomyopathy is the most common cardiac symptom in patients with PCD, and early diagnosis can prevent complications. Next-generation sequencing can identify genetic variants attributable to PCD efficiently.
Objective: We aimed to detect the genetic cause of the early manifestations of hypertrophic cardiomyopathy and metabolic abnormalities in an Iranian family.
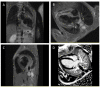
Methods: We herein describe an 8-year-old boy with symptoms of weakness and lethargy diagnosed with PCD through clinical evaluations, lab tests, echocardiography, and cardiac magnetic resonance imaging. The candidate variant was confirmed through whole-exome sequencing, polymerase chain reaction, and direct Sanger sequencing. The binding efficacy of normal and mutant protein-ligand complexes were evaluated via structural modeling and docking studies.
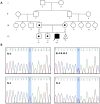
Results: Clinical evaluations, echocardiography, and cardiac magnetic resonance imaging findings revealed hypertrophic cardiomyopathy as a clinical presentation of PCD. Whole-exome sequencing identified a new homozygous variant, SLC22A5 (NM_003060.4), c.821G > A: p.Trp274Ter, associated with carnitine transport. Docking analysis highlighted the impact of the variant on carnitine transport, further indicating its potential role in PCD development.
Conclusions: The c.821G > A: p.Trp274Ter variant in SLC22A5 potentially acted as a pathogenic factor by reducing the binding affinity of organic carnitine transporter type 2 proteins for carnitine. So, the c.821G > A variant may be associated with carnitine deficiency, metabolic abnormalities, and cardiomyopathic characteristics.
Keywords: Cardiomyopathy; Organic cation transporter 2; Primary carnitine deficiency; SLC22A5; Whole-exome sequencing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Therrell BL, Jr, Lloyd-Puryear MA, Camp KM, Mann MY. Inborn errors of metabolism identified via newborn screening: ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol Genet Metab. 2014;113(1–2):14–26. doi: 10.1016/j.ymgme.2014.07.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

