Efficacy of intranasal administration of dexmedetomidine in combination with midazolam for sedation in infant with cleft lip and palate undergoing CT scan: a randomized controlled trial
- PMID: 38166622
- PMCID: PMC10759416
- DOI: 10.1186/s12871-023-02397-2
Efficacy of intranasal administration of dexmedetomidine in combination with midazolam for sedation in infant with cleft lip and palate undergoing CT scan: a randomized controlled trial
Abstract
Background: There is a great challenge to sedation for infants with cleft lip and palate undergoing CT scan, because there is the younger age and no consensus on the type, dosage, and route of drug administration.
Objective: This study aimed to evaluate the efficacy of intranasal administration of dexmedetomidine combined with midazolam as a sedative option for infants with cleft lip and palate under imaging procedures.
Methods: Infants scheduled for cleft lip and palate repair surgery were randomly assigned to the IND group (intranasal dexmedetomidine 2 µg/kg alone) and the INDM group (intranasal dexmedetomidine 2 µg/kg combined with midazolam 0.05 mg/kg). The primary outcome was the proportion of infants underwent successful computed tomography (CT) scans under intranasal sedation. The secondary outcomes included onset time and duration of sedation, recovery time, Ramsay sedation scale, hemodynamic parameters during sedation, and adverse events. Data analyses involved the unpaired t-test, the repeated-measures analysis of variance test, and the continuity correction χ2 test.
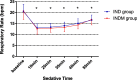
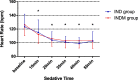
Results: One hundred five infants were included in the analysis. The proportion of infants underwent successful CT scans under sedation was significantly greater in the INDM group than in the IND group (47 [95.9%] vs. 45 [80.4%], p = 0.016). Additionally, the INDM group had a shorter onset time and a longer duration of sedation statistically (12 [8.5, 17] min vs. 16 [12, 20] min, p = 0.001; 80 [63.6, 92.5] min vs. 68.5 [38, 89] min, p = 0.014, respectively), and their recovery time was significantly longer (43 [30, 59.5] min vs. 31.5 [20.5, 53.5] min, p = 0.006). The difference in Ramsay sedation scale values 20 min after administration was statistically significant between the groups. No statistically significant difference was found between the groups in changes in heart rate and respiratory rate.
Conclusion: Intranasal administration of dexmedetomidine in combination with midazolam resulted in higher sedation success in comparison with sole dexmedetomidine. However, it has a relatively prolonged duration of sedation and recovery time.
Trial registration: ChiCTR2100049122, Clinical trial first registration date: 21/07/2021.
Keywords: Cleft lip and palate; Dexmedetomidine; Intranasal sedation; Midazolam.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The effect of age on outpatient pediatric procedural sedation with intranasal dexmedetomidine and oral midazolam.Eur J Pediatr. 2024 Jan;183(1):169-177. doi: 10.1007/s00431-023-05240-5. Epub 2023 Oct 19. Eur J Pediatr. 2024. PMID: 37855928 Free PMC article.
-
Oral midazolam vs. intranasal dexmedetomidine plus oral midazolam for sedation of pediatric outpatients: a double-blinded randomized controlled trial.BMC Anesthesiol. 2023 Oct 10;23(1):341. doi: 10.1186/s12871-023-02289-5. BMC Anesthesiol. 2023. PMID: 37817075 Free PMC article. Clinical Trial.
-
Comparison of oral midazolam with intranasal dexmedetomidine premedication for children undergoing CT imaging: a randomized, double-blind, and controlled study.Paediatr Anaesth. 2017 Jan;27(1):37-44. doi: 10.1111/pan.13010. Epub 2016 Oct 13. Paediatr Anaesth. 2017. PMID: 27734549 Clinical Trial.
-
[Efficacy and safety of intranasal dexmedetomidine premedication for children undergoing CT or magnetic resonance imaging: a systematic review and meta-analysis].Zhonghua Er Ke Za Zhi. 2020 Apr 2;58(4):314-318. doi: 10.3760/cma.j.cn112140-20191224-00830. Zhonghua Er Ke Za Zhi. 2020. PMID: 32234139 Chinese.
-
Intranasal dexmedetomidine versus oral chloral hydrate for diagnostic procedures sedation in infants and toddlers: A systematic review and meta-analysis.Medicine (Baltimore). 2020 Feb;99(9):e19001. doi: 10.1097/MD.0000000000019001. Medicine (Baltimore). 2020. PMID: 32118711 Free PMC article.
Cited by
-
Four-year review of safe and effective procedural sedation in neonates and young infants.Front Pharmacol. 2024 Jul 26;15:1381413. doi: 10.3389/fphar.2024.1381413. eCollection 2024. Front Pharmacol. 2024. PMID: 39130634 Free PMC article.
-
Midazolam for sedation of infants in the neonatal intensive care unit.Cochrane Database Syst Rev. 2025 Jul 17;7(7):CD002052. doi: 10.1002/14651858.CD002052.pub4. Cochrane Database Syst Rev. 2025. PMID: 40673402
-
Paediatric sedation with intranasal dexmedetomidine: Protocol for a systematic review and meta-analysis.PLoS One. 2025 Jan 13;20(1):e0317406. doi: 10.1371/journal.pone.0317406. eCollection 2025. PLoS One. 2025. PMID: 39804873 Free PMC article.
References
-
- Lewis J, Bailey CR. Intranasal dexmedetomidine for sedation in children; a review. J Perioper Pract. 2020;30(6):170–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical