Clinical application of vancomycin TDM in ventilated patients with gastrointestinal cancer: a propensity-matched analysis
- PMID: 38166695
- PMCID: PMC10759445
- DOI: 10.1186/s12879-023-08885-7
Clinical application of vancomycin TDM in ventilated patients with gastrointestinal cancer: a propensity-matched analysis
Abstract
Background: Therapeutic drug monitoring (TDM) of vancomycin is widely recommended for clinical treatment. Due to the complexity of 24-h area under the curve (AUC) guided vancomycin monitoring in clinical practice, the vancomycin trough level remains the most common and practical method. The purpose of this study was designed to investigate the differences in the safety and efficacies of vancomycin TDM based on the two different monitoring methods, and further explore the clinical application of trough-guided vancomycin monitoring in patients with gastrointestinal cancer requiring mechanical ventilation.
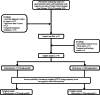
Methods: We included a total of 78 gastrointestinal cancer patients who required mechanical ventilation due to various diseases. All patients included in this study were aged 18 years or older and were treated with intravenous vancomycin therapy for more than 2 days due to documented or suspected Gram-positive bacterial infections, and have at least one available vancomycin plasma concentration. First, we compared the safety and efficacies of vancomycin TDM based on different monitoring methods as trough-guided monitoring or AUC-guided monitoring. Then, based on whether the initial vancomycin concentration achieving the target trough concentration (less than 48 h), patients were divided into early and delayed groups, and the clinical factors were compared between them. The primary endpoints include the incidence of new-onset acute kidney injury (AKI) or renal replacement therapy (RRT), clinical success rate and 28-day all-cause mortality. Finally, the overall relationship between trough concentration and potential covariates is screened by univariate and multivariate analysis to explore potential information covariates.
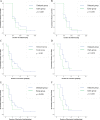
Results: The research revealed that patients with gastrointestinal cancer exhibited significantly lower initial vancomycin trough concentrations (median [interquartile range (IQR)]: 6.90[5.28-11.20] mg/L). And there were no statistically significant differences in the safety and efficacies of vancomycin TDM based on the two different monitoring methods for the primary endpoint. Moreover, base on trough-guided vancomycin monitoring, the early group demonstrated a notably shorter duration of mechanical ventilation compared with the delayed group (χ2 = 4.532; p < 0.05; Fig. 2E). Propensity score weighting further confirmed that the duration of mechanical ventilation (χ2 = 6.607; p < 0.05; Fig. 2F) and duration of vasoactive agent (χ2 = 6.106; p < 0.05; Fig. 2D) were significantly shorter in the early group compared with delayed group. Multivariate regression analysis revealed that Cystatin C (Cys-C) was the most important variable for vancomycin target trough achievement (odds ratio, 5.274; 95% CI, 1.780 to 15.627; p = 0.003).
Conclusions: Trough-guided vancomycin monitoring is a simple and effective marker of TDM for ventilated patients with gastrointestinal cancer. Timely achievement of target trough concentrations for vancomycin can improve partial clinical outcomes in Gram-positive bacterial infections. Cys-C level is a potentially valuable parameter for predicting the vancomycin concentration.
Keywords: Gastrointestinal cancer; Therapeutic drug monitoring; Vancomycin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere JP, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102(6):966–971. doi: 10.1038/sj.bjc.6605578. - DOI - PMC - PubMed
-
- Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–864. doi: 10.1093/ajhp/zxaa036. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

