Anlotinib alone or in combination with bevacizumab in the treatment of recurrent high-grade glioma: a prospective single-arm, open-label phase II trial
- PMID: 38166698
- PMCID: PMC10763299
- DOI: 10.1186/s12885-023-11776-4
Anlotinib alone or in combination with bevacizumab in the treatment of recurrent high-grade glioma: a prospective single-arm, open-label phase II trial
Abstract
Background: Anlotinib is a multi-target tyrosine kinase inhibitor (TKI) targeting the vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and c-Kit. This phase II study aimed to assess the efficacy and safety of anlotinib, either alone or in combination with bevacizumab (Bev) for recurrent high-grade glioma (rHGG) (NCT04822805, 30/03/2021).
Methods: Eligible patients had a histological diagnosis of rHGG with first or subsequent recurrences. All patients received oral anlotinib 12 mg or 10 mg on days 1-14 (repeated every 21 days). In cases where brain magnetic resonance imaging examination revealed an increase in peritumoral edema without worsening of symptoms, patients received a temporary treatment of intravenous bevacizumab 10 mg/kg to alleviate edema. The primary endpoint was the median progression-free survival (mPFS), and the secondary endpoints included median overall survival (mOS), objective response rate (ORR), disease control rate (DCR), and safety.
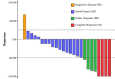
Results: Twenty-five patients with rHGG were included in the efficacy and safety assessments. Eighteen patients received anlotinib alone, and seven patients received anlotinib in combination with Bev. For all patients, the mPFS and mOS were 5.0 months and 13.6 months, respectively. The ORR was 32%, and the DCR was 96%. It is noteworthy that the survival and response data of recurrent glioblastoma (rGBM) exhibit similarities to those of rHGG. For rGBM patients, there were no significant differences in mPFS, mOS, ORR, or DCR between the anlotinib alone and anlotinib + Bev groups. However, the incidence of treatment-related adverse events of any grade was higher in the anlotinib + Bev group compared to the anlotinib alone group (100% vs. 78%, p = 0.041).
Conclusions: Both anlotinib alone and its combination with Bev demonstrated good efficacy and safety in the treatment of rHGG.
Keywords: Anlotinib; Bevacizumab; Multi-target tyrosine kinase inhibitor; Recurrent high-grade glioma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Anlotinib for Patients With Metastatic Renal Cell Carcinoma Previously Treated With One Vascular Endothelial Growth Factor Receptor-Tyrosine Kinase Inhibitor: A Phase 2 Trial.Front Oncol. 2020 May 7;10:664. doi: 10.3389/fonc.2020.00664. eCollection 2020. Front Oncol. 2020. PMID: 32457838 Free PMC article.
-
Anlotinib as Monotherapy or Combination Therapy for Recurrent High-Grade Glioma: A Retrospective Study.Clin Med Insights Oncol. 2023 Jul 6;17:11795549231175714. doi: 10.1177/11795549231175714. eCollection 2023. Clin Med Insights Oncol. 2023. PMID: 37435019 Free PMC article.
-
Phase II study of anlotinib in combination with oxaliplatin and capecitabine for patients with RAS/BRAF wild-type metastatic colorectal adenocarcinoma as the first-line therapy.BMC Med. 2022 May 6;20(1):155. doi: 10.1186/s12916-022-02357-6. BMC Med. 2022. PMID: 35513832 Free PMC article. Clinical Trial.
-
Efficacy and safety evaluations of anlotinib in patients with advanced non-small cell lung cancer treated with bevacizumab.Front Pharmacol. 2022 Sep 27;13:973448. doi: 10.3389/fphar.2022.973448. eCollection 2022. Front Pharmacol. 2022. PMID: 36238567 Free PMC article.
-
Use of Bevacizumab in recurrent glioblastoma: a scoping review and evidence map.BMC Cancer. 2023 Jun 14;23(1):544. doi: 10.1186/s12885-023-11043-6. BMC Cancer. 2023. PMID: 37316802 Free PMC article.
Cited by
-
Off-label use of anlotinib in malignancies' treatment: efficacy and management of adverse reactions.Pharmacol Rep. 2025 Apr;77(2):392-408. doi: 10.1007/s43440-025-00700-1. Epub 2025 Feb 3. Pharmacol Rep. 2025. PMID: 39899257 Free PMC article. Review.
-
Reviewing the path to balance: mechanisms and management of hypertension associated with targeting vascular endothelium in cancer therapy.Hypertens Res. 2025 Mar;48(3):1034-1047. doi: 10.1038/s41440-024-02086-8. Epub 2025 Jan 16. Hypertens Res. 2025. PMID: 39820066 Review.
-
Ascites production and prognosis after ventriculoperitoneal shunt for diffuse midline gliomas in children: A case series.Medicine (Baltimore). 2024 Oct 4;103(40):e39977. doi: 10.1097/MD.0000000000039977. Medicine (Baltimore). 2024. PMID: 39465699 Free PMC article.
-
Treatment mechanism and research progress of bevacizumab for glioblastoma.Am J Cancer Res. 2025 Apr 25;15(4):1874-1901. doi: 10.62347/RNUE7193. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371151 Free PMC article. Review.
References
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a Randomized Clinical Trial. JAMA. 2017;318:2306–16. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

